http://www.chemistrymag.org/cji/2006/087047pe.htm |
Jul. 1, 2006 Vol.8 No.7 P.47 Copyright |
Zhao Baowei, Jiang Bing, Dong Wenjuan, Zhu
Kun
(School of Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou
730070, China)
Received Apr. 3, 2006; Supported by the National Natural Science Foundation of China (20577018), and the Qinglan Talent Project of Lanzhou Jiaotong University.
Abstract A novel spectrofluorimetric determination method of trace amounts of sulphides was described on the basis of Hg (II)-2-(2
'-hydroxylphenyl) benzoimidazole (HPBI) fluorescence quenching system. The fluorescence intensity was linear with the concentration of sulphide in the range of 1.0×10-8 - 9.5×10-6 mol/L. The detection limit was down to 9.02×10-9 mol/L. Interferences was avoided by using standard distillation procedure. The main advantages, apart from the extremely high sensitivity of the method, were the high stability of the reacted sulphide system. The method was also applied to measure the trace amounts of sulphides in water sample with satisfactory results.Keywords 2-(2' -hydroxylphenyl)benzoimidazole, mercury (II), spectrofluorimetry, sulphide 1. INTRODUCTION
The extreme toxicity of hydrogen sulphide is produced through the great ability of sulphide ion reacting with many metals in the human metabolism. Sulphides in water are oxygen demand substance, which consume the dissolved oxygen and can restrain the activity of aquatic creature [1]. The water containing sulphides causes the roots of plant to decay by irrigation. The increase in the concentration of this species in water is mainly attributed to the indiscriminate discharge of inadequate treated effluents of organic matter from industrial wastes as well as the bacterial reduction of sulphate results in the release of sulphide into wastewater. The toxic nature of H2S has claimed several lives, especially of those working in the sewerage systems[2,3]. Therefore, the facts mentioned above lead to a requirement for sensitive analytical methods for the determination of sulphides. The quantitative determination of sulphide in different types of samples has been reported by employing a range of analytical techniques which include iodometry, spectrophotometry, anodic stripping voltammetry, reciprocal oscillographic chronopotentiometry, ion chromatography, enthalpimetry, chemiluminescence, gravimetry, ion selective electrode and gas chromatography [4-18]. The samples analyzed in these experiments ranged from water, wastewater, food materials to inorganic compounds. Spectrofluorimetry is widely used to detect the trace amounts of substances due to its high sensitivity and selectivity.
To our knowledge, rare studies on the fluorescent determination of sulphides were reported in recent years. It has been previously reported that the determination of mercury in wastewater based on the fluorescence quenching system of mercury (II)-2-(2'-hydroxylphenyl)benzoimidazole (HPBI). In the further study, mercury (II) sulphide was formed and equivalent amount of the fluorescent organic ligand was released if the sulphide ion was allowed to enter the system. Thus, the spectrofluorimetric system for the determination of sulphides was established.
2. EXPERIMENTAL
2.1 Apparatus
Fluorescence measurements were performed on a RF-540 Spectrofluorimeter (Shimadzu,
Japan) equipped with a xenon light source and quartz cells of 1 cm pathlength. The
excitation and emission slits were both 10 nm. The pH values of the solutions used were
measured with PHS-2 Acidity Meter (Shanghai, China).
2.2 Reagents
Standard stock solution of mercury (II) (1.0×10-3 mol/L) was prepared
by dissolving 0.2006 g of pure mercury with concentrated nitric acid, heating the solution
to dry, adding 2 drops of concentrated nitric acid and diluting the solution to 1000 mL.
This solution was standardized with EDTA. 2-(2' -hydroxylphenyl) benzoimidazole (HPBI) was
synthesized according to literature
2.3 Sampling and storage
The samples were preserved by adding 0.2 mL (4 drops) of 2 mol/L zinc acetate and 0.05 mL (1 drop) of 6 mol/L sodium hydroxide to a 100-mL polyethylene bottle, which was completely filled with the sample and was stoppered. If the concentration of sulphide was greater than approximately 100 mg/L, the volume of both regeants added to each 100 mL sample was increased.
2.4 Procedures
To a series of 100 mL flasks, 0.8 mL of mercury standard stock solution, 1.0 mL of HPBI solution and 5.0 mL of buffer solution were added and diluted to a volume of about 60 mL. Then appropriate amount of standard solution of sodium sulphide or the sample solution treated by the standard distillation procedure was added, and the solution was diluted to 100 mL and mixed well. The intensity of fluorescence of solution was measured at excitation wavelength of 320 nm and emission wavelength of 430 nm.
3. RESULTS AND DISCUSSION
3.1 Optimum conditions
It is evident that the excitation and emission wavelength of the reagent HPBI is at 320nm and 430nm respectively. After the complex of HPBI with mercury (II) is formed, the excitation and emission wavelength of HPBI is not changed. However, its fluorescent intensity of emission largely decreases. The further study showed that not only the excitation and emission wavelength did not change, but also the intensity of emission at 430 nm quenched by mercury (II) was regained because mercury (II) sulphide was formed while sulphide ion was mixed into system. Therefore, 320 nm and 430 nm were chosen as the excitation and emission wavelength respectively, at which the fluorescent intensity of solutions was measured (Fig. 1).
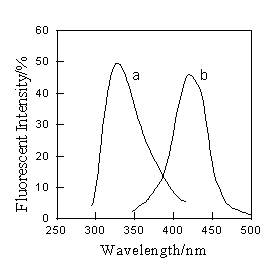
Fig.1 Fluorescent spectra
(a: excitation spectra; b: emission spectra)
Acidity plays an important role in this system. A change in pH value of solution causes a change in fluorescent intensity. Consequently the pH value of solution was controlled strictly by addition of Na2B4O7-NaOH buffer solution. It is found that the intensity maintains maximum and constant in the pH range from 9.6 to 10.5 (Fig. 2). When the optimum addition of the buffer solution was determined as 2.0 mL, a pH value of 10.0 was therefore adopted in the following procedure.
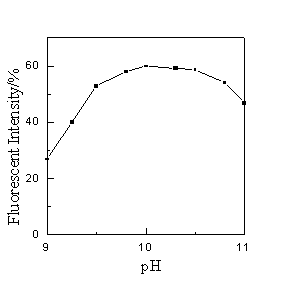
Fig.2 Effect of acidity on the fluorescent Intensity
The presence of
surfactants could affect the intensity of system. Various kinds of surfactants were tested
and it was found that the addition of surfactant resulted in a slighter decrease of
intensity, perhaps because the stability of complex of mercury (II) with HPBI increased,
which prevented mercury from reacting with sulphide ion.
HPBI is slightly dissolvable in water. Its working solution was
prepared in ethanol as mentioned previously. The addition of HPBI solution was controlled
strictly since the presence of ethanol could greatly enhance the intensity of system.
At the optimum conditions, the complexing reaction of mercury with HPBI
occurred instantly and the fluorescent intensity of system was constant within 10 h after
the addition of sulphide ion.
The calibration graph for the determination of sulphide was constructed
under the optimum conditions. Excellent linearity was obtained over the concentration
range 1.0×10-8 - 9.5×10-6 mol/L of sulphides. The
limit of detection was down to 9.02×10-9 mol/L. Corresponding
regression results were obtained as: F(%)=5.5 + 8.5×106 C (mol/L), R=0.9978,SD=0.0075,N=11, where F is the fluorescent intensity of the system and C the
concentration of sulfide ion.
The effect of foreign ions on the fluorescent intensity of system was
studied for 2.0×10-6 mol/L of sulphide ion. Given tolerance did not
cause 5 % deviation. The results showed that the oxidizing ions, such as ClO3-,
BrO3- and IO3-, interfered the
fluorescence seriously. However, sulphide ion does not co-exist with these ions in water
body. Metal ions or heavy metal ions caused the fluorescent intensity to change, but their
interferences can be avoided by the standard distillation. The anions, which can enter the
alkaline absorption solution with hydrogen sulphide in the distillation procedure, were
tested. The results are shown as follows: F-, Cl-, Br-,
I- (500), C2O42-, HPO32-,
HSO4- (100),CN-
(60),SCN- (50). Here tolerance
times are notified in brackets.
Table 1 Analysis results of wastewater samples (n=6)
Samples |
Values of Determination |
Addition of Standard Sulphide |
Values after addition of Standard sulphide |
Recovery |
1 |
0.875 |
3.20 |
4.01 |
98.1 |
2 |
0.417 |
6.40 |
6.72 |
98.5 |
3.2 Sample analysis and results
By this method, two kinds of wastewater containing sulphides were detected. The
results are shown in Table 1. If the presence of interfering substances is suspected or if
the analysis has to be delayed, the sulphide is fixed by allowing it to react with zinc
acetate.
4. CONCLUSIONS
The studied method for the determination of sulphide in water has a high sensitivity
and a high selectivity and takes less time. It can be utilized to determine trace amounts
of sulphides in wastewater. Its application in environmental monitoring for atmospheres
and anaerobic sediments will be continued.
REFERENCES
[1] Borum J, Pedersen O, Greve T M et al. The Journal of Ecology, 2005, 93 (1):
148-158.
[2] Whiteman M, Cheung N S, Zhu Y Z et al. BioChem.Biophys.Res.Commun., 2005, 326 (4):
794-798.
[3] Bhatia M, Sidhapuriwala J, Moochhala S M et al. Bristish Journal of Pharmacology,
2005, 145 (2): 141-144.
[4] Safavi A, Ramezani Z. Talanta. 1997, 44 (7): 1225-1230.
[5] Ceba M R, Jara F V, Leyva J A M. Analyst, 1982, 107 (1276): 781-786.
[6]Krishnamarty M, Srinivasa R N V. Analyst, 1983, 108 (1282), 119-122.
[7] Kester M D, Shiundu P M, Wade A P. Talanta, 1992, 39 (3): 299-312.
[8] Koh T, Okabe K. Analyst, 1994, 119 (11): 2457-2461.
[9] Wandruszka R V, Yuan X, Morra M J. Talanta, 1993, 40 (1): 37-42.
[10] Hu J, Zhu J, Zheng I et al. Analytical Laboratory (Fenxi Shiyanshi), 1994, 13 (1),
30-32.
[11] Rucklin R D, Johnson E L. Anal.Chem., 1983, 55 (1): 4-7.
[12] Kiba N, Nishijma M, Furusawa M. Talanta, 1980, 27 (12): 1090-1092.
[13] Burguera J L, Townshend A. Talanta, 1980, 27 (4): 309-314.
[14] Teckentrup J, Klockow D. Talanta, 1981, 28 (9): 653-663.
[15] Padma D K. Talanta, 1986, 33 (7): 550-552.
[16] Hiti I K A, Moody G J, Thomas J D R. Analyst, 1983, 108(1282): 43-52.
[17] Hara H, Okazai S. Analyst, 1984, 109 (10): 1317-1320.
[18] Balasubramanian S, Pugalenthi V. Water Research, 2000, 34 (17): 4201-4206.
[19] Mishra A, Singh M P, Singh J P. J. Indian Chem. Soc., 1980, 27, 249-251.
赵保卫, 蒋兵, 董雯娟,朱琨
(兰州交通大学环境与市政工程学院, 兰州730070)
摘要 本文以Hg (II)- 2-(2'-羟基苯基)苯并咪唑荧光体系为基础, 建立了水样中痕量硫化物的荧光分析法. 荧光强度与硫化物浓度呈线性关系, 线性范围1.0×10-8 ~ 9.5×10-6 mol/L, 检测限为9.02×10-9 mol/L. 以蒸馏法消除共存物质干扰. 方法灵敏度高,反应体系稳定, 用于水样中痕量硫化物分析, 得到较好结果.
关键词 2-(2'-羟基苯基)苯并咪唑, 汞(II), 荧光光度法, 硫化物