http://www.chemistrymag.org/cji/2006/087050pe.htm |
Jul. 1, 2006 Vol.8 No.7 P.50 Copyright |
(School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China) Received Mar.10, 2006;Supported by the Natural Science Foundation of China (20573136), the Natural Science Foundation of Guangdong province (04009715) and the team project of the Natural Science Foundation of Guangdong province (04205405).
Abstract The electrochemical window of acetamide-urea-NaBr-KBr melt is 2.00 V. The melt is a good medium for the deposition of rare earth-iron group alloys. The electroreduction of Co(II) and Nd(III) in this melt were studied by cyclic volatmmetry and chronoamperometry. The reduction of Co(II) is an irreversible process. The transfer coefficient
of Co(II) reduction was calculated to be 0.16 and the diffusion coefficient of Co(II) was calculated to be 0.28¡Á10-10 cm2·s-1. Nd(III) can not be reduced alone, but Nd-Co can be codeposited by induced deposition. The Nd content in Nd-Co film varies with the potential and the molar ratio of Nd(III)/Co(II), the maximum Nd content is Nd (77.08 wt%)-Co deposited at -1.25 V. The morphology of Nd-Co film was investigated by SEM and AFM. Nd-Co film comprises of nanoparticles, the size is from 50 to 100 nm. XRD shows it is amorphous. After heat-treatment at 1153 K the crystal Nd2Co17 phase was formed. The magnetic properties of the Nd-Co films were determined using hysteresis loops, the coercive field Hc of Nd (77.08 wt%)-Co amorphous film is 700 Oe at 5K, the remanent magnetization MR and the saturation magnetization MS are 0.27 and 1.64 emu·g-1 respectively.Keywords Nd-Co amorphous film, nanoparticles, induced codeposition, urea melt, magnetic properties
1. INTRODUCTION
The films of Rare Earth-Iron Group (RE-IG) alloys have many functional performances
and have been widely applied in mechanical, magnetic, optical, battery, superconducting
materials and devices [1-5]. Electrodeposition has been proved to be a good way
to prepare amorphous [6] and nanoparticle [7] alloy films.
Electrodeposition in inorganic electrolyte [8-9] and in aqueous electrolyte [10]
have been reported. However the deposits contain oxygen which is inevitable during
electrolysis in the solution having water. For example the Sm-Co alloys derived from
aqueous solution contain a relevant amount of oxygen and the maximum content of oxygen
reaches up to about 50at%[10].
In order to reduce the environmental pollution, non-volatile ionic
liquids and near ambient temperature organic molten salts have been recently utilized as
potential substitution of traditional organic solvents for catalytic synthesis, separation
and electrochemical process [11]. The systems of urea with some alkali metal
salts have lower eutectic point from 28 to 116oC [12], greater
solubility of metal chloride, and higher conductivity [13]. The urea melt can
also dissolve some metal oxides on the electrode surface and remove impurity water by
itself [14]. The electrodeposition of many RE-IG alloys in urea-NaBr-KBr melt
at 100 oC have been reported by authors[15]. Acetamide has high
dielectric constant and can easily dissolve many inorganic salts. By addition of acetamide
to urea-NaBr-KBr, the melting point will further lower. Hence the acetamide-urea-NaBr-KBr
melt is suitable to be used as the medium for electrodeposition of RE-IG alloys. In this
paper, the induce codeposition of Nd-Co alloy films in acetamide- urea-NaBr-KBr melt at 70oC
and the magnetic properties of Nd-Co films were investigated.
All chemical reagents used are analytically pure. SmCl3 was prepared by the reaction of Sm2O3 and HCl (36.5%) and then was evaporated and dehydrated at HCl atmosphere under vacuum. CoCl2 was obtained by the dehydration of CoCl2·6H2O under vacuum at 393 K for more than 10 h. Urea was also dried under vacuum at 393 K. The mixture of urea, acetamide and the salts was melted at a temperature lower than 353 K. The experimental temperature was controlled at 343 K by oil bath thermostat.
The electrochemical cell was a H-shape electrobath in which the anode and cathode chamber were isolated by sintered glass. The working electrode was a Pt film (99.9%, 10.28mm2), the counter electrode Pt plate (99.9%, 20.51 cm2). The reference electrode was an Ag wire deposited with AgCl at its surface and immersed in urea-acetamide-NaBr melt in a glass tube, which has a Luggin capillary filled with asbestos fiber at the tip. The potential of this reference electrode was calibrated with reference to a SCE electrode. All the potentials in this paper are given with reference to the SCE electrode.
The electrochemical measurements were performed by a voltalab 80 universal electrochemical instrument (Radiometer Analytical) under Ar atmosphere. Pre-electrolysis was carried out to remove residual water and other impurities. Nd-Co alloy films were deposited on a Cu plate by potentiostatic electrolysis. The as-deposited samples were washed by DMSO to remove urea and salts adhered to the surface. The Cu plate deposited with Nd-Co film was cut into two pieces, each piece was put into a crucible placed in a quartz tube and subsequently undergone heat treatment at Ar atmosphere.
The phase and structure of both the samples were determined by X-ray diffraction (XRD, D/MAX-3A; Tigaku International Corp. ltd) using Cu Ka line at 40 KV 40 mA. The composition of the alloys was analyzed by EDAX (X-ray Energy Dispersive Analysis) with a Link-ISIS-300 (Oxford). The surface morphology was examined by scanning electron microscope (SEM, S-520; Hitachi) and atomic force microscope (AFM, SPM-9500J3, Shimadzu). Magnetic properties were measured by superconductive quantum magnetometer (MPMS XL-7, QUANTUM DESIGN).
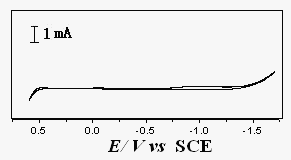 Fig.1 CV of
a Pt electrode in the background melt at 343K, 100 mV·S-1
Fig.1 CV of
a Pt electrode in the background melt at 343K, 100 mV·S-1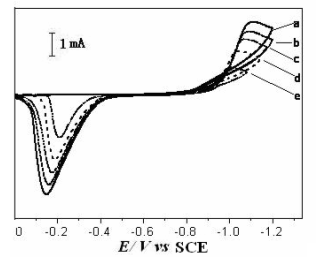
Fig.2 CV of a Pt (0.10 cm2) in 0.02 mol·L-1 CoCl2-acetamide-urea-NaBr-KBr at 343 K
n/ mV·s-1: (a) 100, (b) 70, (c) 50, (d) 30, (e) 10 3. RESULTS AND DISCUSSION
3.1 Electrochemical behaviors of Co(II) and Nd(III) in acetamide-urea-NaBr-KBr melt
The acetamide-urea-NaBr (1.43¡Á10-3 mol·m-3)-KBr (1.1¡Á10-4 mol·m-3) was used as the background melt. Fig.1 is the cyclic voltammogram (CV) of a Pt electrode in the background melt. The cathodic limit is -1.40 V and the anodic limit is 0.60 V, thus the electrochemical window of this low temperature molten salt is 2.00 V.
Fig. 2 shows the cyclic voltammogram (CV) of a Pt in CoCl2-acetamide-urea-NaBr-KBr melt. At the scan rate of 100 mV·s-1, the cathodic wave appears at -0.85 V and the peak potential is -1.05 V. After reversal sweep, an anodic current was observed at -0.40 V. The potentiostatic electrolysis was carried out at -1.05 V and the Co film with silvery white metallic luster was obtained. Therefore this cathodic peak corresponds to the electroreduction of Co(II) to Co, while the anodic peak is due to the stripping of Co. The cathodic peak potential shifts negatively with the increase of the scan rate v and the plot of Ip ~ v1/2 is linear. Thus the electroreduction of Co(II) to Co is irreversible in one step. The positive delayed loop can be observed in Fig. 2. It suggests that the electrode process is controlled by nucleation [16].
For an irreversible electrode process, the relationship between peak potential Ep and scan rate v is[17]:
and the relationship between peak current Ip and scan rate v is[17]:
According to the slope of equation (1) and (2), the transfer coefficient can be calculated to be 0.16 and the diffusion coefficient of Co(II) D is 0.28¡Á10-10 cm2·s-1.
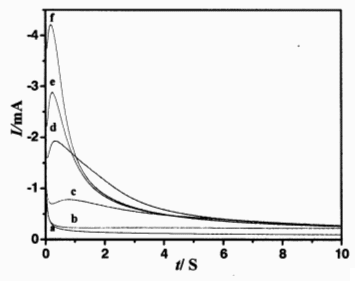
Fig.3 I-t curves of a Pt (0.10 cm2) in 0.02 mol·L-1 CoCl2-acetamide-urea-NaBr-KBr at 343 K
E/V: (a) -0.8, (b) -0.9, (c) -1.0, (d) -1.1, (e) -1.2,(f)-1.3
Fig. 3 is the chronoamperogram of Pt electrode in CoCl2-acetamide-urea-NaBr-KBr melt. The Faradaic current occurred at the step potential of -0.9 V or more negative than -0.9 V, this is in accord with the result obtained from CV. The current went up and dropped down after the maximum because of the growth of nucleus and the formation of the new phase. When the mass transfer reached the limit, the concentration of the electroactive species on the electrode surface was nearly zero and the transient current kept decreasing. At this stage the diffusion-limited current, id, obeys the Cottrell equation:
According to equation (3) the diffusion coefficient of Co(II) D was calculated to be 0.64¡Á10-10 m2·s-1.
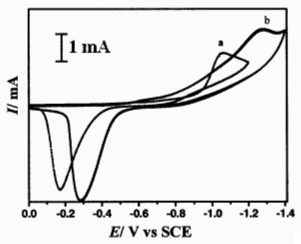
Fig. 4 CV of a Pt in (a).CoCl2 (0.02 mol·L-1)-acetamide-urea-NaBr-KBr melt and (b) CoCl2(0.02 mol·L-1)- NdCl3 (0.1 mol·L-1)-acetamide-urea-NaBr-KBr, 343 K, 100 mV·s-1
3.2 Inductive codeposition of Nd-Co alloy
Because of the negative reduction potential of rare earth ions the rare earth metal can not be deposited in urea low temperature melt. Fig. 4 shows the CV of a Pt electrode in NdCl3-CoCl2-acetamide-urea- NaBr-KBr melt. Obviously the cathodic peak current in curve b is larger than that in curve a. This implies that the induced codeposition of Nd(III) and Co(II) occurred. The potentiostatic electrolysis was performed at -1.30 V on a copper substrate at 343K.
The Nd-Co films were obtained by potentiostatic electrolysis on a copper substrate in 0.1 mol·L-1 NdCl3-0.02 mol·L-1CoCl2-acetamide-urea-NaBr-KBr melt at different potentials from -1.10 V to -1.30 V. It was found that the maximum content of Nd is Nd (77 wt%)-Co which was obtained at -1.25 V. At -1.25 V the variety of the Nd-Co composition with the molar ratio of Nd(III)/Co(II) was investigated and the results are listed in Table 1. It can be seen that the Nd content in Nd-Co film increases with increasing molar ratio of Nd(III)/Co(II).
Table 1 The influence of Nd(III)/Co(II) molar ratio on the composition of Nd-Co films
Nd(III)/Co(II) |
3:1 |
4:1 |
5:1 |
Co /wt% |
33.75 |
24.69 |
22.92 |
Nd /wt% |
66.25 |
75.31 |
77.08 |

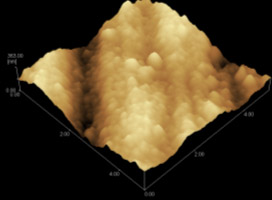
Fig. 5 SEM and AFM images of Nd-Co film
deposited at -1.30 V
3.3 Structure of Nd-Co film
The morphology of Nd-Co film is shown in Fig. 5. It can be found that
the film consists of a lot of small particles which is nano sized from 50 to 100 nm. The
size and the distribution of these particles can be clearly seen although they are not
discrete. The formation of the nano sized nucleus may be caused by the induced
codeposition. Schwartz et al.[18] investigated the codeposition of Sm-Co alloy
in aqueous containing glycine and pointed out that the codeposition involves hetero-nuclear
glycinato coordination complexes. The complexes adsorbed on the cathode provide step-wise
reduction of the depositing metals with surface adsorbed H atoms and/or direct
electron transfer, resulting in alloy deposits. It can be thought that the Nd-Co induced
codeposition may also involve Co(II) and Nd(III) hetero-nuclear urea coordination
complexes. We further inference that the codeposition of Nd-Co originates from the
reduction of Co(II) in the coordination complexes. Co(II) obtains electrons and then is
partly reduced to Co. The electrons obtained by Co(II) can also be transferred to RE(III)
through the conjugated chain in the complex and Nd(III) is reduced to Nd. This is why the ip
in curve (b) is higher than that in curve (a) in Fig. 4. Because of the dependence and
competition of the electroreduction of Nd(III) and Co(II) the growth of Nd-Co nucleus will
be interdicted, especially when the reduction of Co(II) predominates the electrode
reaction. Therefore the nano sized particles is formed.
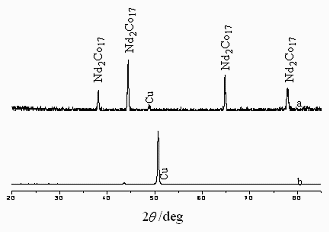
Fig.6 XRD patterns of Nd-Co film (a) before heat treatment; (b) after
heat treatment at 1173 K
Fig. 6 shows the XRD
patterns of Nd-Co film before (curve b) and after (curve a) heat-treated. Except for the
diffraction peak of the Cu substrate, no other diffraction peaks are found on curve b.
This indicates that the film is amorphous. The formation of the amorphous film is also
caused by the induced codeposition [19]. After heat-treatment at 1173 K, Nd2Co17
diffraction peaks appear on curve a.

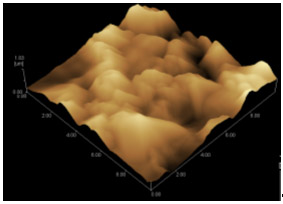
Fig.7 SEM and AFM images of Nd-Co film deposited at -1.30 V after heat treatment
at 1173 K for 3 h
Fig. 7 is the SEM and AFM images of Nd-Co film after heat treatment at
1173 K. The nano sized particles disappeared and large crystal bulk can be found. This is
in accord with the result obtained from the XRD pattern.
Table 2 Magnetic parameters of Nd-Co films
Nd /wt% |
T /K |
Hc /Oe |
Mr/ emu·g-1 |
Ms / emu·g-1 |
77.08 |
5 |
700 |
0.27 |
1.64 |
77.08 |
293 |
150 |
1.40 |
9.23 |
19.97 |
5 |
400 |
0.37 |
1.89 |
19.97 |
293 |
250 |
0.15 |
0.57 |
77.08* |
5 |
290 |
2.40 |
14.21 |
77.08* |
293 |
275 |
1.82 |
8.79 |
*Heat-treatment at 1173.15 K for 3 h and
cooling down naturally
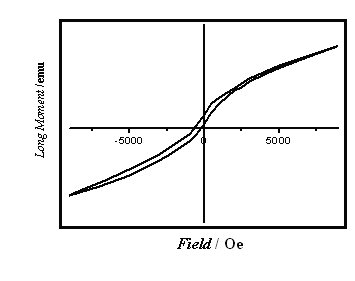
Fig. 8 The magnetic hysteresis curves of Nd(77.08wt %)-Co alloy measured
at 5 K, before heat-treatment
3.4 The magnetic properties of Nd-Co film
Fig. 8 is the hysteresis loop of Nd(77.08wt %)-Co before heat-treatment. The coercive
field Hc is 700 Oe, the remanent magnetization MR and
the saturation magnetization MS are 0.27 and 1.64 emu·g-1
respectively. The magnetic parameters of Nd-Co alloys before and after heat treatment are
summarized in Table 2.
It can be seen from Table 2 that the coercive field Hc
is high. The difference of the coercive field Hc at 5 K and 293 K is
remarkable before heat-treatment, but not apparent at 5 K and 293 K after heat-treatment.
For amorphous materials, at low temperature the coercive field of the alloy with
high Nd content is larger than that with low Nd content; on the contrary, at high
temperature the coercive field of the alloy with high Nd content is less than that
with low Nd content. The values in Table 2 comply with this regularity.
The magnetic properties depend on the composition of the alloy, the
more the rare earth content is the higher the coercive field is [20]. In Table
2 the coercive field is affected by composition, at 5 K the coercive field is higher when
the Nd content is high.
(1)The electrochemical window of acetamide-urea-NaBr-KBr melt is 2.00 V.
(2)The electroreduction of Co(II) is an irreversible process. The transfer coefficient of Co(II) reduction was calculated to be 0.16 and the diffusion coefficient of Co(II) is 0.28¡Á10-10 cm2·s-1.
(3)Nd(III) can not be reduced alone, but Nd-Co can be codeposited by induced deposition.
(4)The Nd content in Nd-Co film varies with the potential and the molar ratio of Nd(III)/Co(II), the maximum content is Nd (77.08 wt%)-Co deposited at -1.25 V.
(5)Nd-Co film comprises of nanoparticles, the size of the particles is from 50 to 100 nm. XRD shows it is amorphous.
(6) The crystal Nd2Co17 phase was formed after the amorphous Nd-Co film was heat-treated at 1153 K.
(7)The coercive field Hc of Nd (77.08 wt%)-Co amorphous film is 700 Oe at 5K, the remanent magnetization MR and the saturation magnetization MS are 0.27 and 1.64 emu·g-1 respectively.
(8) The coercive field of the amorphous Nd-Co film varies greatly with the temperature, while the effect of the temperature to coercive field of the crystal Nd-Co film is not remarkable.
REFERENCES
[1] N. Godshall, J Electrochem. Soc.
[2] F. J. Di Salvo, Science, 1990, 285: 703.
[3] J.J. Wyslocki, P. Pawlik, W. Kaszuwara, M. Leonowicz, Journal of Magnetism and Magnetic Materials, 2004, 272¨C276: e1929.
[4] L.A. Dobrza'nski, M. Drak, Journal of Materials Processing Technology, 2004, 157-158: 650.
[5] D. Raasch, H. Wierenga, Journal of Magnetism and Magnetic Materials, 1997, 168: 336.
[6] L. Wang, J. Peng, L. Tang, Rare Earth Mater. and Eng. 2004, 33: 113.
[7] D..J. Riley, Current Opinion in Colloid & Interface Science, 2002, 7: 186.
[8] P. Liu, Q. Q. Yang, Y. S. Yang, Y. X. Tong, Journal Rare Earths, 1999, 17: 151.
[9] P. Liu, Q. Q. Yang, Y. X. Tong, Y. S. Yang, Electrochimica Acta, 2000, 45: 2147.
[10] J Zhang, Paul Evans, Giovanni Zangari, J. Magn. Magn. Mater. 2004, 283: 89-91.
[11] Liao Ju-Hsiou, Wu Pei-Chi, Bai Yi-Hsuan, Inorg. Chem. Communication, 2005, 8: 390.
[12] M. Gambino, J.P. Bros and L. Ter Minassian, Thermochimica Acta,1988, 127: 223.
[13] Q. Q. Yang, G..K. Liu and P. Liu, Acta Scientiarum Naturalium Universitatis Sunyatsen, 1995, 34: 47.
[14] E.M. Golubchik and Z. Prikl. Khim, 1989, 62: 2229.
[15] Q. Q. Yang, Electrochemistry,1997, 3: 117. (in Chinese).
[16] G. J. Hills, D. J. Schiffrin and J. Thompson, Electrochimica Acta, 1974, 19: 657-670
[17] A.J. Bard, L.R. Faulkner (Eds.), Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 1980, 257, 298, 166. (in Chinese).
[18] M. Schwartz, N.V. Myung, K. Nobe, J. Electrochem. Soc., 2001, 51 (4): C468-C477.
[19] H. Somekawa, et al, Instrumented indentation properties of electrodeposited Ni¨CW alloys with different microstructures, Scripta Materialia, 2004, 50: 1361-1365
[20] S. Aich, J.E. Shield, Journal of Magnetism and Materials, 2004, 279: 76. ¡¡