http://www.chemistrymag.org/cji/2006/088054pe.htm |
Aug. 1, 2006
Vol.8 No.8 P.53 Copyright |
Determination of norfloxacin using an electrochemically pretreated glassy carbon electrode
Sun Hanwen, Xing Tao, Lian Kaoqi, Liang Shuxuan(College of Chemistry and Environmental Science, Hebei University, Key Laboratory of Analytical Science and Technology of Hebei Province, Baoding 071002, China)
Received Apr. 22, 2006; Supported by the Specialized Research Funds of China Education Ministry (No.20050075003)
Abstract The electrochemical behaviors of norfloxacin on a pretreated glassy carbon electrode were investigated by cyclic voltammetry. The results indicated that the process was irreversible and fundamentally controlled by adsorption. The solution conditions and instrumental parameters were investigated and optimized. An optimal curve of norfloxacin was got in 0.1 mol/L phosphate buffer solution of pH 5.29 at 100 mV/s after 2.0 min stirring at 0.3 V. Norfloxacin gave a sensitive adsorptive oxidative peak at 1.17 V (versus Ag-AgCl). The peak current was in linear relation to the norfloxacin concentration in the range of 0.5-5.0 mg/mL. The detection limit was 0.2 mg/mL. This method was used for determination of norfloxacin in capsule,and the RSD was below 2.0 %. Chromatography analysis was also carried out to confirm this method. Statistical analysis of the results using Student t-test and the variance ratio F-test showed no significant difference (p=0.95) between the performance of the two methods as regards to accuracy and precision.Keywords Norfloxacin; Glassy carbon electrode; Electrochemical pretreatment; Capsule analysis 1. INTRODUCTION
Norfloxacin[NFLX]{1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolonecarboxylic acid}, is one of fluoroquinolone synthetic antibiotics. The chemical structure is shown in Fig.1.
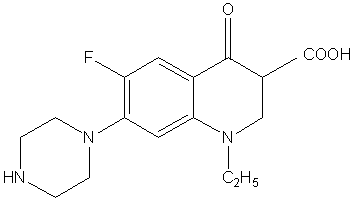
Fig.1 Chemical structure of norfloxacin
This group of drugs is bactericidal over a wide range of therapeutically achievable concentrations and acts via selective inhibition of bacterial DNA synthesis. NFLX has a broad range of action against both gram negative and gram positive bacteria. It is widely used in the treatment of respiratory tract and urinary tract infections [1].
Many methods had been developed for the determination of NFLX, such as HPLC [2-5], high-performance thin-layer chromatography [6], flow-injection chemiluminescence [7, 8], spectrophotometric determination [9-11]. A solid-phase luminescence test method for the determination of ciprofloxacin and NFLX had been described with the detection limit of 1 mg/mL for NFLX [12].
During the past few years several papers on the polarographic behavior of NFLX had appeared in the literatures, including polarographic investigation of the redox mechanism and the application of adsorptive stripping voltammetry at the hanging mercury drop electrode for the determination of NFLX [13-16]. A PVC membrane-NFLX selective electrode based on NFLX iodide and bismuth iodide molecular association complex had been reported with the detection limit of 4.5 ×10-5 mol/L [17]. Based on the polarographic and voltammetric behavior of Mn(II)-NFLX complex,the change of peak height with the concentration of NFLX was linear in the range from 8.0×10-6 to 1.9×10-5 mol/L[18]. The adsorptive and electrochemical behavior of NFLX on a glassy carbon electrode had been investigated by cyclic and square-wave voltammetry with detection limit of 1.1 mg/mL urine [19].
In this paper, a new method for determination of NFLX was described by cycle volammetry using a modified glassy carbon electrode (GCE) with the detection limit of 0.2 mg/mL.
2. EXPERIMENTAL
2.1 Apparatus
Voltammetric measurements were performed with MEC-12B Multi-function Microcomputer
Electrochemical Analyzer (Jiangsu Jiangfen Instrument Int., China) and ATA-IB Rotating
Disk Electrode. The three-electrode system consisted of a glassy carbon electrode, Ag-AgCl
(saturated KCl ) reference electrode and a platinum wire auxiliary electrode. The KQ218
ultrasonic instrument (Kunshan Ultrasonic Instrument Factory, China) was used. A high pure
water machine XGJ-30 (Beijing Yong Cheng Water Treatment Technology Co., LTD) was used to
prepare pure water.
Chromatography was performed by using a Shimadzu HPLC system with UV
detection (Shimadzu, Kyoto, Japan). This system was equipped with a photodiode array
detector (SPD-M10Avp), a gradient controller (SCL-10Avp), a workstation (CLASS-vp), a
degasser (DGU-12A) and a column thermostat (CTO-10Avp). An ODS column (Shimadzu, Kyoto,
Japan 150×4.6mm i.d) was used.
2.2 Chemicals
The standard NFLX sample was supplied by National Institute for the Control of
Pharmaceutical and Biological Products. NFLX capsule was obtained from the drugstore. KH2PO4
and Na2HPO4 were purchased from Beijing Yi Li Fine Chemicals Co.,
Ltd (Beijing China). KNO3 was purchased from Tianjin Fuchen Chemical Reagent
Factory. CH3CN was purchased from Tianjin Kermel Chemical Reagents Development
Center. CH3OH was purchased from Tianjin Reagent Chemical Products Co., Ltd.
Phosphate buffer [PB] solutions at different pHs were got by mixing 0.1mol/L KH2PO4
and 0.1 mol/L Na2HPO4 at different ratios.
All the chemicals used were of analytical reagent grade except HPLC
grade reagents for HPLC analysis, and all solutions were prepared with pure water.
2.3 Pretreatment of the GCE
The glassy carbon electrode (GCE) was polished with 0.3 and 0.05 mm aluminum powders, until a mirror-like
finish was obtained. After washed with pure water under the aid of ultrasonication, the
electrode was oxidized at +1.80 V for 5min in 0.1 mol/L PB solution (pH 5.0) and scanned
between + 0.3 and + 1.4 V until current-potential curve was steady.
2.4 Capsule analysis
Three series of single capsule of NFLX were dissolved in 100 mL 0.1 mol/L HCl, and
filtrated to get clear liquor. Then they were diluted by 0.1 mol/L PB solution (pH 5.29)
to the suitable concentration and 15.0 mL solution was transferred into a voltammetric
cell. After stirring at 0.3 V for 2.0 min, the pretreated GCE was scanned at the rate of
100 mV/s from 0.3 V to 1.4 V. Quantification was performed by means of calibration curve
method. To make a comparison, another assay was carried out by HPLC according to Chinese
Pharmacopoeia [20].
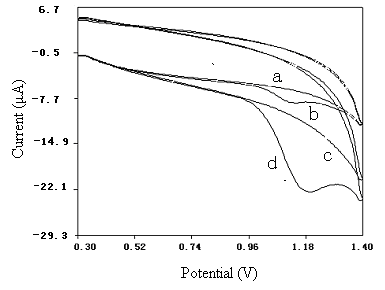
Fig.2Cyclic voltammetric sweeps in 0.1 mol/L
pH 5.29 PB solution (blank a, c ; and 2.0 mg/mL NFLX b, d ) at unpretreated GCE (a, b) and pretreated GCE (c,
d).Accumulated at +0.3 V for 2 .0 minutes and scanned at 100 mV/s.
3 RESULTS AND DISCUSSIONS
3.1 Cyclic voltammetric speciality
A new phase on GCE could be formed by electrochemically anodized at +1.80 V in 0.1mol/L
PB, which contained a significant amount of microcrystallinity and graphite oxide. The
characteristic of the new phase was porous and hydrated, and it appeared that the
pretreatment affected primarily the accumulation process and not the charge transfer
process.
Fig.2 shows the cyclic voltammograms of 0.1 mol/L PB solution at pH
5.29. As can be seen, for the un-pretreated GCE, the CV exhibited a broad peak with poor
current response for NFLX oxidation. In contrast, for the pretreated GCE, an enhancement
in the oxidation peak current was observed at 1.17 V.
Compared with the un-pretreated GCE, the pretreated GCE had better catalytic activity for
the oxidation of NFLX. Only an oxidation peak of NFLX was
observed at the pretreated GCE, it showed that the redox process of NFLX on the pretreated
GCE was irreversible.
NFLX molecule has p electron conjugated system, which was adsorbed to the surface of
the electrode. In pH 5.5-9.5 NFLX was adsorbed mainly as neutral molecular
adsorption type, but RCOO- form was increased with the increase of pH value, so
neutral molecular adsorption type would be transformed to anion adsorption type [21].
It was suggested that NFLX in PB solution (pH 5.29) was adsorbed mainly as
neutral molecular adsorption type. An anodic peak observed on pretreated GCE was probably
caused by the oxidation of piperazine ring in the molecule [22].
3.2 Effect of supporting electrolyte and pH
Different supporting electrolytes were investigated, namely, CH3COOH-CH3COONa,
KCl, Na2B4O7·10H2O, HNO3,
KNO3, and PB. The results showed that PB could give a best peak, thus, the PB
was chosen as the supporting electrolyte.
The electrode process was related with [H+]. Both the peak
potential and peak current depended on pH value of solution, as shown in Fig.3 and Fig.4.
The pH dependence of oxidation peak potential of NFLX obeyed the equation,
Ep(V)=1.568-0.076 pH (r=0.998). The peak potential is 1.17 V for pH
5.29.The peak current decreased with increasing pH value of solution. Considering the peak
current and the shape of the peak, pH 5.29 was chosen.
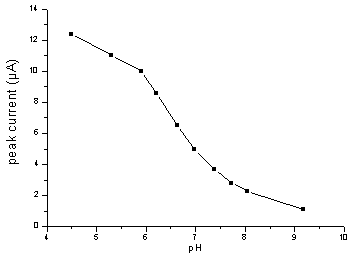
Fig.3 Effect of pH on the peak current for
2.0 mg/mL NFLX in 0.1 mol/L
PB solution (a certain pH) at pretreated GCE. Accumulated at +0.3 V for 2.0 minutes and
scanned at 100 mV/s.
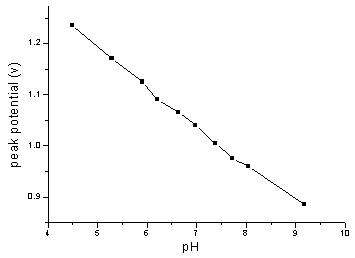
Fig.4 Effect of pH on the peak potential for
2.0 mg/mL NFLX in 0.1 mol/L
PB solution (a certain pH) at pretreated GCE. Accumulated at +0.3 V for 2.0 minutes and
scanned at 100 mV/s.
3.3 Effect of scan rate
Effects of scan rate on the peak current and the peak potential were investigated, as
shown in Fig. 5 and Fig. 6.The results showed that peak current increased with increasing
scan rate. The peak current was linear with scan rate in the range of 100-300 mV/s. It
obeyed the equation,Ip(mA)=4.36157+0.0445u(mV/s), which showed the electrode process was controlled by
absorption. Meanwhile, increasing scan rate could cause the peak potential moving towards
anodic position and high background current. Scan rate of 100 mV/s was suitable for
determination.
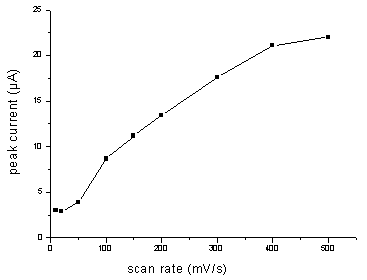
Fig.5 Effect of scan rate on the peak
current for 2.0 mg/mL NFLX
in PB solution ( pH 5.29) at pretreated GCE. Accumulated at +0.3 V for 2.0 minutes and
scanned at a certain rate.
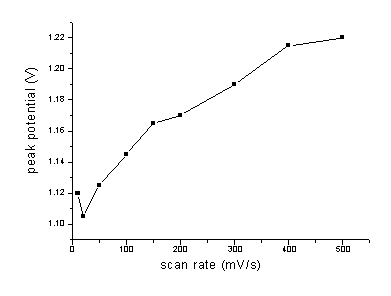
Fig.6 Effect of scan rate on the peak
potential for 2.0 mg/mL
NFLX in 0.1 mol/L PB solution (pH 5.29) at pretreated GCE. Accumulated at +0.3 V for 2.0
minutes and scanned at a certain rate.
3.4 Effect of the enriching time and
potential
The enriching process could affect the oxidation. Fig.7 indicated that the peak
current grew as time grew during 0.5-2.0 min. When the time continued to lengthen, the
increase of the peak current was not obvious, which means the adsorption reached the
saturation. Fig.8 showed that the peak current decreased with increasing enriching
potential but at low enriching potential, the shape of the peak was well. +0.3 V was
chosen as enriching potential and 2.0 min as enriching time, which could give an optimum
result.
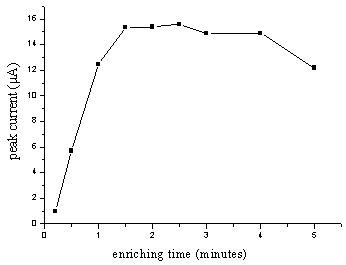
Fig.7 Effect of enriching time on the peak
current for 2.0 mg/mL NFLX
in 0.1 mol/L PB solution (pH 5.29) at pretreated GCE. Accumulated at +0.3 V for certain
minutes and scanned at 100 mV/s
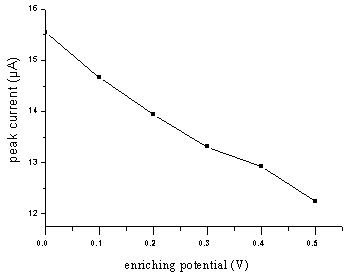
Fig.8 Effect of the enriching potential on
peak current for 2.0 mg/mL
NFLX in 0.1 mol/L PB solution (pH 5.29) at pretreated GCE. Accumulated at certain voltage
for 2.0 minutes and scanned at 100 mV/s.
3.5 Determination of capsule sample
The peak current was linear with the NFLX concentration in the range of 0.5-5.0 mg/mL. The detection limit was 0.2 mg/mL. This method was suitable
for determination of NFLX in capsule. The content of NFLX in capsule was determined by
HPLC according to Chinese Pharmacopoeia in order to confirm the results obtained by
pretreated GCE. Three kinds of NFLX capsule from different factory were chosen to carry on
the experiment. The results were shown in Table 1. Statistical analysis of the results
using Student t-test and the variance ratio F-test showed no significant difference
(p=0.95) between the performance of the two methods as regards to accuracy and precision.
Table 1 Determination of NFLX in capsule samples
Sample |
Theoretical content |
Proposed method |
HPLC method |
||
(mg/tablet) |
Content |
RSD |
Content |
RSD |
|
1 |
100 |
100.42±1.47 |
1.46 |
102.24±1.66 |
1.62 |
2 |
100 |
102.98±1.73 |
1.68 |
98.54±1.91 |
1.94 |
3 |
100 |
110.74±1.61 |
1.45 |
108.42±0.95 |
0.88 |
4. CONCLUSION
A new phase on glassy carbon electrode (GCE) could be formed by electrochemically
anodized at +1.80 V in 0.1 mol/L phosphate buffer solutions (PB). It could effectively
enhance the current of NFLX on the GCE. The process of norfloxacin (NFLX) on this
electrode was irreversible and fundamentally controlled by adsorption. Norfloxacin gave a
sensitive adsorptive oxidative peak at 1.17 V (versus Ag-AgCl) in phosphate buffer solution (pH 5.29). The peak current was
in linear relation to the norfloxacin concentration in the range of 0.5-5.0 mg/mL. The detection limit was 0.2 mg/mL. The method could be developed to
determine the NFLX in capsule. Chromatography analysis was also carried out to confirm
this method. Statistical analysis of the results using Student t-test and the variance
ratio F-test showed no significant difference (p=0.95) between the performance of the two
methods as regards to accuracy and precision.
REFERENCES
[1] Zhang W S, Li A L. Medicinal Chemistry. Beijing: Higher Education Press, 1999:545.
[2] Mascher H J, Kikuta C. J. Chromatogr.A, 1998, 812: 381.
[3] Du L M, Wei H Q, Zhang J Y, et al. Chinese J. Chromatogr. (Se Pu), 2003, 21(5): 503.
[4] Samanidou V F, Demetriou C E, Papadoyannis I N. Anal. Bioanal. Chem., 2003, 375: 623.
[5] Rao R N, Nagaraju V. J. Pharmaceut. Biomed., 2004, 34: 1049.
[6] Simonovska B, Andrens?k S, Vovk I, et al. J. Chromatogr.A, 1999, 862: 209.
[7] Liang Y D, Song J F, Yang X F. Anal. Chim. Acta., 2004, 510: 21.
[8] Ma H Y, Zheng X W, Zhang Z J. Chinese J. Anal. Chem., 2004, 32(7): 857.
[9] El Walily A F M, Belal S F, Bakry R S, J. Pharmaceut. Biomed., 1996, 14: 561.
[10] Chen H, Zheng D H, Journal of Taizhou University (Taizhou Xueyuan Xuebao), 2004,
26(3): 64.
[11] Wang H Q, Huang Z Z, Zhang L. PTCA (PART B: CHEM. ANAL) (Lihua Jianyan) 2005, 41: 35.
[12] Beltyukova S, Teslyuk O, Egorova A, et al. J. Fluoresc, 2002, 12(2): 269.
[13] Jaber A.M.Y, Lounici A, Anal. Chim. Acta, 1994, 291: 53.
[14] Jaber A M Y, Lounici A. Analyst, 1994, 119: 2351.
[15] Wang X L, Zhang S M, Zhang W W, et al. Anal.Lett , 1996, 29(1): 131.
[16] Wang X L, Zhang S M, Zhang W W, et al. Chinese J. Anal. Chem. (Fenxi Huaxue), 1995,
23(10): 1189.
[17] Li D H,Wang M, Ding Y D. Chinese J. Anal.
Chem.(Fenxi Huaxue), 1996, 24(8): 931.
[18] Zhang S M, He C X, Jiang J Y,et al. Chinese J. Anal. Chem.(Fenxi Huaxue), 2004,32(3):
332.
[19] Ghoneim M M, Radi A, Beltagi A M, J. Pharmaceut Biomed, 2001, 25: 205.
[20] China Pharmacopoeia Committee. Chinese Pharmacopoeia. 2000 Edition. Beijing:
Chemistry Industry Press, 2000: 753.
[21] Liu X J, Chen G N, Xie D C, et al. Chin. J. Pharm. Anal. (Yaowu Fenxi Zazhi), 1995,
15(2): 30.
[22] Warowna-Grzeskiewicz M, Chodkowski J, Fijalek Z, Acta. Pol. Pharm., 1995, 52(3):187.
孙汉文 邢涛 连靠奇 梁淑轩
(河北大学化学与环境科学学院,河北省分析科学技术重点实验室,071002, 保定)
摘要 本文采用循环伏安法研究了诺氟沙星在预处理玻碳电极上的电化学行为。结果表明,该过程是一个不可逆并且由吸附控制的过程。在0.1mol/L磷酸盐缓冲溶液(pH=5.29)中,0.3 V富集2.0 min,然后以100 mV/s的速度从0.3 v扫描到1.4 V,诺氟沙星在1.17 V (相对于Ag/AgCl,饱和KCl)有一灵敏的吸附氧化峰,并且峰电流与诺氟沙星浓度在0.5-5.0mg/mL内呈线性关系,检出限为0.2mg/mL。本方法可以用于胶囊中诺氟沙星的测定,相对标准偏差低于2.0%,通过采用色谱法进行对照实验,进一步证明该方法的可靠性和准确度。
关键词 诺氟沙星 玻碳电极 电化学处理 胶囊测定