http://www.chemistrymag.org/cji/2006/088053pe.htm |
Aug. 1, 2006 Vol.8 No.8 P.53 Copyright |
Preparation and characterization of hydroxyapatite/chitosan-sodium alginate composite materials
Lu Caixia, Yao Zihua, Li Zhiyun, Wang Yunke(Research Center of Physical and Chemistry Analysis, Hebei University; Key Laboratory of Analytical Science and Technology of Hebei Province, Baoding 071002, China)
Abstract Hydroxyapatite/Chitosan-sodium alginate nanocomposite materials were prepared through co-precipitation method. The mechanical performance, component and morphology of the composites were determined by means of UTM, XRD, TEM, SEM and TGA. Results indicated that nano-hydroxyapatite particles having similar chemical composition to nature bone in the composites were dispersed evenly in Chitosan and sodium alginate matrix. The mechanical property of the nanocomposite was well with maximum compressive strength value of 47.183 MPa at 50wt% HA of HA/CS-Alg. The composites can meet the demands of repairation and substitution of bone tissue and have potential application prospect in internal fixation of bone fracture. Furthermore, the research was helpful for the theoretical development of bone tissue engineering.
Keywords Hydroxyapatite; chitosan; sodium alginate; composite; co-precipitation method
1. INTRODUCE
Bone repair or regeneration is a common and complicated clinical problem in
orthopaedic surgery. Hence ideal bone substituted material have been widely studied.
Nature bone is actually an inorganic/organic composite mainly consisted of nano-structure
hydroxyapatite (Ca10(PO4)6(OH)2,HA) and
collagen fibers[1]. Hydroxyapatite is a major inorganic component of bone and
one of excellent candidates for bone repair and regeneration, because of its similar
chemical composition to that of the mineral phase of bone. It has been used extensively
for biomedical implant applications and bone regeneration due to its bioactive,
biodegradable and osteoconductive properties [2,3]. Owing to its inadequate
mechanical properties and brittleness, the instability of the HA particulate is often
encountered when the particles are mixed with saline or patient's blood, making it
unable to be used as a load bearing implant.
To solve the problem, incorporation of HA into polymer matrix has
also been carried out to increase osteoconductivity and biodegradability with significant
enhancement of mechanical strength [4]. Recently, a great attentions have been
focused on the composite of HA in conjugation with natural polymers [5,6] and
synthetic polymers [7,8]. Chitosan (CS), an inexpensive, inert, hydrophilic,
biocompatible linear polymer, derived from the exoskeletons of insects and shells of
crustaceans, is the second most abundant natural polysaccharide after cellulose. The
chemical structure of chitosan is b-(1,4)-2-amino-2-deoxy-D-glucose which is obtained by partial
deacetylating chitin. Due to its unique properties such as biodegradability, non-toxicity,
anti-bacterial effect and biocompatibility [9], chitosan have been used widely
in a variety of biomedical application, such as enzyme carriers, wound dressing, drug
delivery carriers, tissue engineering, bone healing materials [10,11], now much attention has been paid to chitosan-based biomedical
materials. Besides, alginate (C5H7O4COONa, Alg) is
another polysaccharide occurring in large amounts in nature, which is extracted from
seaweeds. It is composed of a 1-4-linked block polymer of polyglucuronate (poly-G),
polymannuronate (poly-M) acids or its copolymer bound in a random sequences[12].
Sodium alginate and chitosan are ionic polymer of polysaccharides, which have good
biocompatibility, high bioactivity and bonding properties.
2.1 Materials and Instruments
Biomedical grade Chitosan(CS) with 99 per cent degree of the deacetylation; analytical grade calcium nitrate tetrahydrate (Ca(NO3)2·4H2O); Potassium di-hydrogen Phosphate (KH2PO4); sodium alginate was purchased from Tianlian Refined Co. Ltd. Shanghai, China; acetic acid; an injectable syringe with needle diameter of 0.7mm; a magnetic stirrer; Instron 1185 Universal Testing Machine (UTM); JEM-100SX (Japan electron) transmission electron microscopy (TEM); KYKY-2800B scanning electron microscopy (SEM) with a Thermo Noran-Vantage X-ray energy dispersive spectrometer; Y-2000 X-ray diffraction (XRD); Pyris6 thermogravimetry (TGA) made in PerkinElmer.
2.2 Preparation of hydroxyapatite/chitosan-sodium alginate composite materials
The solution of Ca(NO3)2 and KH2PO4 were prepared with near 1.67 Ca/P stoichiometric ratio. The 2 wt﹪chitosan solution is prepared by dissolving chitosan into 2 wt﹪ acetic acid with stirring on a magnetic stirrer for 4h to get a perfectly transparent solution. The 2wt﹪sodium alginate solution is prepared by dissolving the deionized water with stirring on a magnetic stirrer for 4h. The homogeneous sodium alginate solution was injected in the chitosan solution by a syringe with needle diameter of 0.7mm, the mass ratio of chitosan and sodium alginate is 2:1. The Ca(NO3)2 solution was added into the mixture of chitosan and sodium alginate solution with vigorous stirring for 10h, and pH was adjusted with ammonia solution to about 9-10 at 40ºC, then the KH2PO4 solution was dropped slowly into the mixture. The reaction temperature was maintained at 40ºC, the stirring was kept for 10h. The obtained white slurry was aged for 24h, and then the precipitate was filtered. In order to avoid serious aggregation of ultrafine powder during drying, the water in the precipitate was replaced by ethanol, the stirring was kept for 10h, filtered again, washed two to three times with distilled water. The precipitated composites were filled in a mould and then dried in a vacuum oven at 80ºC.
2.3 Characterization of the hydroxyapatite/chitosan-sodium alginate composite materials
The cylindrical blocks of approximately10mm height and 10mm diameter were obtained. The blocks were then determined for its compressive strength by Universal Testing Machine. The composites were characterized by X-ray diffraction with Ni-filtered CuKa radiation generated at 25kV and 30mA at the wavelength of 1.54Å. The dispersion and particle size of composite were evaluated by a transmission electron microscopy. After the samples were cut as cross section by a sharp knife and sputtered with gold, the morphology of the composite was observed under scanning electron microscope. The organic and inorganic contents of composite were determined by using thermogravimetry analysis, about 10mg of samples were heated at the heating rate of 20ºC/min and the measurements were recorded from 50ºC to 800ºC.
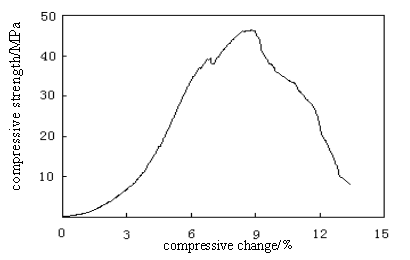
Fig. 1 The maximum compressive strength of HA/CS-Alg composite 3.RESULTS AND DISCUSSION
3.1 Compressive strength
The equipment used for carrying out the test was universal testing machine with an operating head load of 5 KN. Cross-sectional area of the sample of known width and thickness was calculated. The measurements of the compressive strength of the composites were conducted to examine the mechanical performance. As shown in Figure 1 maximum compressive strength value of 47.183 MPa was obtained at 50wt﹪ HA of HA/CS-Alg composite , which indicating that such a novel bioabsorbable composite could be used as internal fixation of bone fracture in the view of application.
3.2 XRD analysis
Fig 2 shows the X-ray diffraction patterns for pure n-HA, chitosan and HA/CS-Alg composite. The characteristic peaks (Fig.2a) for chitosan were detected in the spectrum, two broad peaks of chitosan at 2q =10.1° and 19.8° are observed. The XRD pattern indicated that the inorganic phase was a single phase of HA (Fig.2c) with characteristic diffraction of (002) at 25.9° and with the overlapping diffraction of (300), (211), and (112) at 31.8°. Fig.2b showed the FWHM of a diffraction peak was very broad, which means that the crystallinity of the HA precipitated is lower. The diffraction from chitosan and sodium alginate were not detected from the XRD patterns, which means that the chitisan and sodium alginate obtained have amorphous structure.
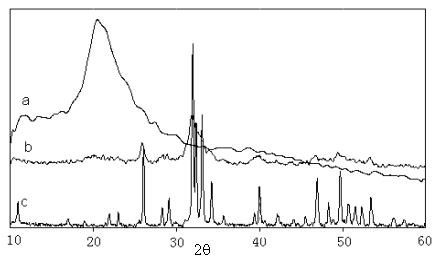
Fig. 2 XRD patterns of (a) chitosan (b) HA/CS-Alg=50:50 composite (c) hydroxyapatite
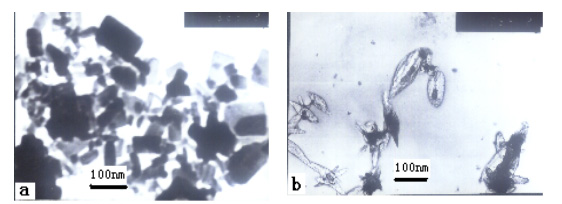
Fig. 3 TEM photographs of (a) pure n-HA (b) HA/CS-Alg=50:50 composite
3.3 TEM measurements
observations
In order to confirm the XRD results concerning the distribution
of HA crystallites in the composite, TEM measurements were performed. TEM photographs of
n-HA and hydroxyapatite/chitosan-sodium alginate composite were shown in Fig.3. The TEM
micrographs revealed the structural features of the composites. By shape, the HA
nano-crystallites were found to be rod-like, with the addition of chitosan, the composite
powders show a slimly shuttle-like morphology with an average of about 80nm in length and
20 nm in width[15]. This infers that the growth of n-HA crystals in the (002)
direction of c-axis may have been promoted. The HA were dispersed in the
chitisan and sodium alginate matrix homogeneously. This advantage may be helpful for
forming uniform block materials, especially when using colloidal processing along with
slip casting [16].
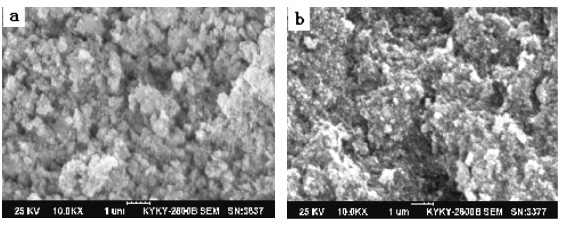
Fig. 4 SEM of (a) pure HA (b) HA/CS-Alg=50:50 composite
3.4 Surface profiling and
SEM
The surface morphological changes of the HA/CS-Alg=50:50 composites were studied by
scanning electron microscope. Fig 4 shows the microstructures of the specimens. Fig 3a
exhibited nano-size HA with almost uniform size particle, though having a number of
micro-cracks and flaws. Fig 3b shows the composite filled with chitsan and sodium
alginate, the HA particles are embedded in the matrix to form tight composite, which
redound to the increase of compressive strength.
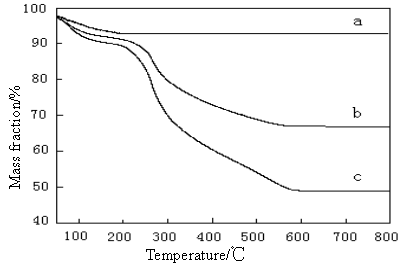
Fig. 5 TGA curves of (a) pure HA; (b) The composite of 30% HA ; (c) The
composite of 50% HA
3.5 TGA analysis
The TGA thermograms of prepared samples are shown in Fig 5. It can be seen from the trace
of HA (Fig 5a) that there is no phase transformation taken place upon heating, which
indicates its thermal stability at high temperature. The observed mass loss at about 120
4. CONCLUSION
In the paper, the composites of HA/CS-Alg
have been prepared by a co-precipitation method. There is an excellent miscibility between
the matrix of chitisan, sodium alginate and n-HA, nano-size HA identified by TEM, SEM and
XRD was perfectly incorporated into the composites. The analysis of TGA indicated the
composition of composite were consistent with as initially added during the preparation,
stronger interactions among the composition have been occurred, which endows the composite
with well mechanical strength. The compressive strength can meet the need of bone carrying
capacity. Based on the excellent properties, the composite of HA/CS-Alg can meet the
demand of bone repairation of osteo-tissue engineering and substitute, and have extensive
potentials to be used as substituted materials in bone tissue engineering and will
contribute to the guided regeneration of new bone.
[1] De Groot K. J Ceram Soc Jpn, 1991, 99: 943-953.
[2] Suchanek W, Yoshimura M. J Mater Sci, 1998, 13: 94-116.
[3] Wei G, Ma PX. Biomaterials, 2004, 25, 4749-4957.
[4] Yamaguchi I, Tokuchi K, Fukuzaki H, et al. J Biomed Mater Res, 2001,55: 20-27.
[5] Zhao F, Yin Y, Lu WW, et al. Biomaterials, 2002, 23: 3227-3234.
[6] Murugan R, Panduranga Rao K. Bioceramics, 2002, 15: 407-410.
[7] Murugan R, Panduranga Rao K. J Biomater Sci Polym Ed, 2003, 14: 457-468.
[8] Murugan R, Panduranga Rao K. Macromol Res, 2003, 11: 14-18.
[9] Va rum KM, Holme HK, Izume M, et al. Biochim Biophys Acta, 1996, 1291(1): 5-15.
[10] Rao S B, Sharma C P. J Biomed Mater Res, 1997, 34: 21-28.
[11] Tarsi R, Muzzarelli RAA, Guzman CA, et al. J Dent Res, 1997, 76: 665-672.
[12] Wasikiewicz J M, Yoshii F, Nagasawa N, et.al. Radiation Physics and Chemistry, 2005, 73(5): 287-295.
[13] Ishihara M, Nakanishi K, Ono K, et al. Biomaterials, 2002, 23: 833-840.
[14] Chatchawalsaisin J, Podczeck F, Newton J M. International Journal of Pharmaceutics, 2004, 275: 41-60.
[15] Viorel M R, Chuen H N, Max W, et.al. Biomaterials, 2005, 26: 5414-5426.
[16] Chen F, Wang Z C, Lin C J. Materials Letters, 2002, 57: 858-861.
[17] Murugan R, Ramakrishna S. Biomaterials, 2004, 25: 3829-3835. 羟基磷灰石/壳聚糖-海藻酸钠复合材料的制备及表征
吕彩霞,姚子华,李志云,王云科
(河北大学理化分析中心,河北省分析科学技术重点实验室 保定 071002)
摘要 应用共沉淀法制备的羟基磷灰石/壳聚糖-海藻酸钠复合材料具有优异的性能。本文应用UTM, XRD, TEM, SEM, TGA等检测方法对材料的成份、形貌、性能等进行了分析表征。结果表明复合材料中的纳米羟基磷灰石类似于自然骨矿物的纳米晶体,并均匀分散于壳聚糖和海藻酸钠的基质中,复合材料具有良好的力学性能,当羟基磷灰石含量为50%时,抗压强度达47.183MPa,可满足骨组织修复和替代的要求,在作为骨折的内部的固定方面有潜在的应用前景。另外,此项研究对骨组织工程的理论发展有一定意义。
关键词 羟基磷灰石;壳聚糖;海藻酸钠;复合材料;共沉淀法