| http://www.chemistrymag.org/cji/2006/089060pe.htm | Sep. 8,
2006 Vol.8 No.9 P.60 Copyright |
Xu Jianzhong1, Wang Chunzheng1, Tang Ranxiao1,2
(1.College of Chemistry and Environmental Science, Hebei University, Baoding ,Hebei 071002, China; 2.College of Science , Agriculture University of Hebei , Baoding, Hebei 071001 , China )
Abstract Complexes of copper (II) with
the ligand synthesized by substituting the chlorine atoms of hexachlorocyclophosphazene
with ethoxy groups have been prepared in different molar ratios. The complexes behavior
was studied preliminarily by infrared spectra, melting point
and thermal analysis.
Keywords Complexes; Copper (II)
chloride; Fire retardant; Phosphazene.
In recent years, many researchers on flame retardants are searching for new composite flame retardants which are environment friendly, high efficiency and multi-function. More attentions have been paid on new inorganic flame retardants, such as phosphorus-nitrogen and silicon containing compounds.
Hexachlorocyclophosphazene, an excellent flame retardant which is one of new phosphorus-nitrogen compounds with special framework. The chlorine atoms on it can be easily substituted by other groups; and the nitrogen atoms with single lone electron pair can coordinate with metals[1]to get a kind of flame retardant complexes which contain phosphazene framework and metal [2].
Roesky et al [3-6] reported the synthesis of some metal derivatives of cyclophosphazene, but little attention has been paid on the complexes of phosphazene with transition metal elements [3]. In view of this, the present paper deals with the preparation and characterization of the complexes of hexachlorocyclophosphazene with copper (II). 2 EXPERIMENTAL
2.1 Materials
Phosphorus Pentachloride (Xinguang Chemical Co., Beijing China), Ammonium chloride (Hebei University Chemical Co., Baoding China), Carbon Tetrachloride, Chlorbenzene, Methanol, Copper Chloride (Huadong Chemical Co., Tianjin China), Methylbenzene, Pyridine (Xintong Chemical Industry, Tianjin China), absolute ethyl alcohol (Beilian Chemical Co., Tianjin China), Metal Sodium (Damao Chemical Co., Tianjin China), n-Heptane (Beichen Huayue Chemical Co., Tianjin China). All the chemicals were purified by standard methods[7] before use. Anhydrous CuCl2 was prepared by baking in oven at 110℃ for 3h.
2.2 Experiment
2.2.1 Synthesis of phosphazene ligands(L)
Phosphorus pentachloride (21.5g, 0.1mol) and ammonium chloride (5.5g,0.1mol) were refluxed in chlorobenzene (500cm3) for 4h. From the reaction mixture, hexachlorocyclophosphazene trimer (NPCl2)3 was crystallised out in n-heptane (50 cm3) as a white solid[8] (m.p. 115℃,78% yield). The mixture of trimer : sodium ethoxide at the ratio of 1 :6 were refluxed in toluene for 1 h to obtain phosphazene ligand(L), a white solid with the yield of 64% . The formula of the ligand was given in Figure 1.
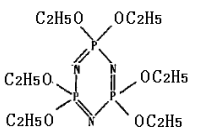
Figure 1 Hex – substituted ligand, N3P3(OC2H5)6 or (L)
2.2.2 Synthesis of the Complexes
A solution of hexaethoxycyclotriphosphazene (4.70g 0.01mol in 20 cm3 benzene)
was added slowly to a solution of copper (II) chloride
(1.62g 0.01mol in 50 cm3 anhydrous methanol). The mixture was stirred and
refluxed for 4h to get a clear green solution. The excess solvent was removed under vacuum
(1.5mm/40℃). The residue was purified from anhydrous
methanol to get a light green sticky solid material with the yield of 30%.
The others complexes were also synthesized following the same procedure
by changing the mole ratio of hexaethoxycyclotriphosphazene : Anhydrous Copper Chloride
to 1:2,1:3.
2.3 Analysis methods
2.3.1 Melting point test
Melting point test was performed at 2℃/min on a XT-4
binocular micro melting point calcimeter, the temperature range is from room temperature
to 200℃.
2.3.2 FTIR analysis
Samples were analyzed by Fourier transform after potassium bromide tabletting on Vertor27
(Bruker).
2.3.3 Thermal analysis
Thermal analysis was performed at 20℃/min on a WCT-2
differential thermal analyzer under N2 .
3.RESULTS AND DISSCUSSIONS
3.1 Reaction equation
(1) The formation of hexachlorocyclophosphazene:
![]()
( Where n = 3、4 or 5 )
The product ( m. p. 115℃ ) was crystallized in
n-heptane
(2) The formation of cyclophosphazene ligand:
![]()
(3) The formation of Complexes of copper(Ⅱ) chloride:
![]()
( Where L is cyclophosphazene ligand and x = 1,2 or 3)
3.2 Influence of water
In the presence of water, phosphazene would decompose into phosphoric acid, ammonia and
hydrochloric acid [9]; and the products would not be gained. Another reason is
that the coordination capability of water to Cu(II) is
better than that of phosphazene. Anhydrous environment is necessary in all the procedure.
3.3 Infrared spectrum
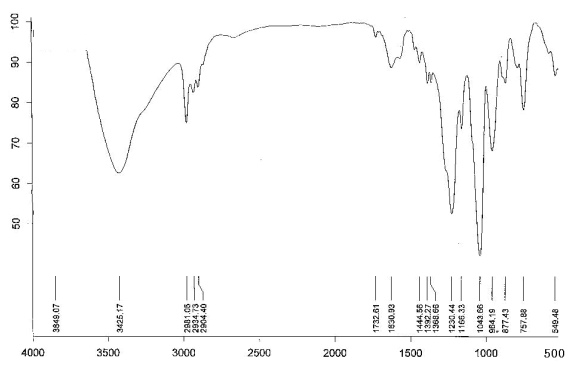
Figure 2 IR spectra of L
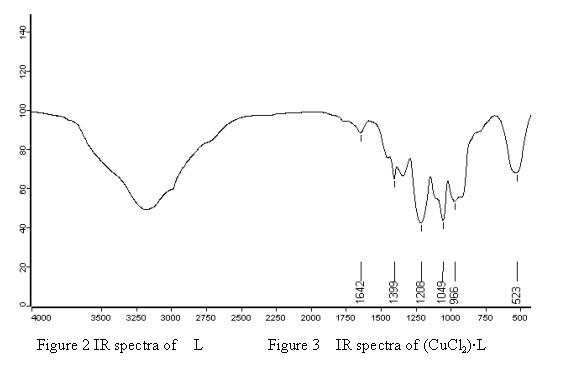
Figure 3 IR spectra of (CuCl2)·L
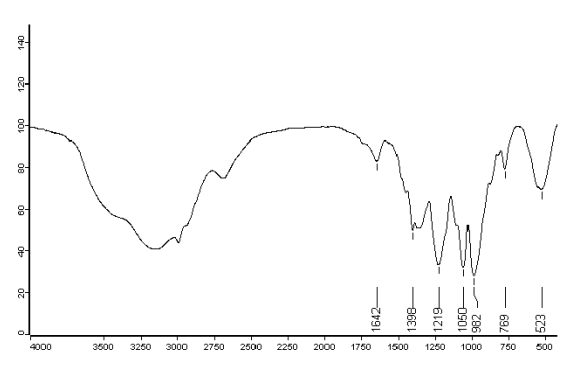
Figure 4 IR spectra of (CuCl2)2·L
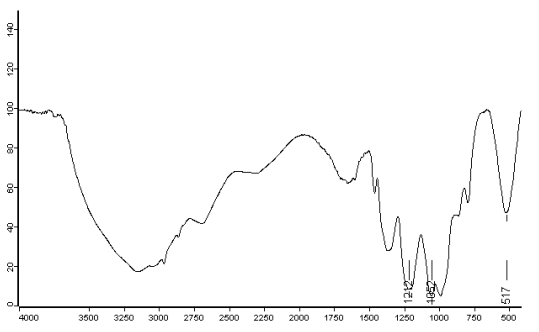
Figure 5 IR spectra of (CuCl2)3·L
Figure 2 was IR spectra of
hexaethoxycyclotriphosphazene, the bands for it were as follows: 3425.17cm-1
was attributed to the stretching vibration of C-H; 2981.05 cm-1, 2934.73 cm-1
were attributed to the resonance of CH2, CH3, which indicated there
were group -CH2 and -CH3 in the product; 1392.25 cm-1, 1444.56 cm-1
were attributed to the stretching vibration of C-C, which proved the presence of -CH2 and -CH3
in the product; 1230.44cm-1 was attributed to the stretching vibration of P=N;
1043.66 cm-1 was the absorption peak of P-O-P, which showed the presence of
P-O-P. The above data were consistent with that of the reference[10]. In the IR
spectra of the cyclotriphosphazene ligand, a strong broad band was observed in the region
1150 - 1300 cm-1 ( 1230cm-1 ), which could be attributed to a degenerate ring n ( P=N ).
In spectra of the complexes, this band shifted to a lower frequency ( 20 - 30 cm-1 ). This
shifting could be attributed to coordinating of phosphazene ring nitrogen to the metal.
The above data were consistent with the reference[11,12].
3.4 Melting point test
The color of the sample changed from light green to brown-green at the temperature range
of 120-140℃, but the sample did not melt; then there
was no change till 200℃.
3.5 Thermal analysis
The result of thermal analysis was given in Figure 6.
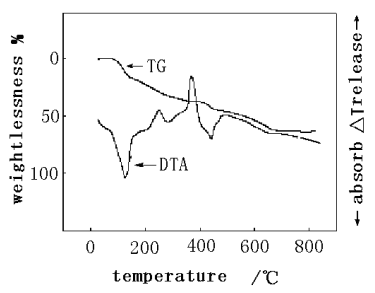
Figure 6 TG-DTA curve of the product
As shown in figure 6, there were three stages in the TG curve of the product, and the starting weight loss temperature of each stage was 98.72℃, 138.8℃ and 442.4℃, with the weight loss rate of 16.45%, 28.48%, 19.61% respectively. There was an endothermic peak at 124.3℃ in the DTA curve, which showed the weight loss was an endothermic process below 125℃, included the volatilization of extraneous small molecules absorbed on the product. Combined the result of melting point meter, the products were found to be decomposed above 124.3℃ and did not melt below this temperature[11]. There were two exothermic peaks at 248.6℃ and 368.6℃,which could be attributed to the decomposition of the phosphazene ring and the continuous decomposition of decomposed product. The exothermic peak at 441.2℃ was attributed to the decomposition and carbonization of organic groups from the product. The weight of product was no longer changed above 666.4℃, and the residual was 37% of initial weight.
4 CONCLUSION1. Hexaethoxycyclotriphosphazene (L) was prepared by –OC2H5 substituting the chlorine atoms of the hexachlorocyclophosphazene. (CuCl2)·L,(CuCl2)2·L and (CuCl2)3·L were synthesized by coordination of copper (Ⅱ) chloride with L .
2. The results of thermogravimetric analysis and melting point meter showed that the products had no melting point, and started to partly decomposed at 120℃.
REFERENCES
[1] Saraceno R.A., Dialing G.H., Allcock H.R., et al., J. Am. Chem. Soc, 1988, 110: 980.
[2] Guglielmi M., Brusatin G., Facchin G. and Gleria M., Appl. Organomet. Chem., 1999,
13(5): 339.
[3] Witt M & Roesky H. W., Chem. Rev, 1994, 94: 1163.
[4] Witt M, Roesky H. W., Stakke D., et al, J. Chem. Soc, Dalton Trans, 1989.
[5] Mulder N. H., Meijers W. H., Sleijfer D. T., et al, Cancer Treat Rep., 1987, 71: 155.
[6] Witt M, Stalke D., Henkel T., et al., J. Chem. Soc, Dalton Trans, 1991.
[7] R. J. Errington, Advanced Practical Inorganic and
Metalorganic Chemistry', Chapman and Hall, London,
1997.
[8] Xu J.Z., He Y.W., et al., J. of Hebei University(Hebeidaxue Xuebao), 2006, 2: 64.
[9] Mujumdar, A.N., et al., Macromolecules, 1990, 23(1): 14.
[10] Zhang J.S., Synthesis and Study of Polyphoaphazenes and Their Derivatives, Qingdao
University(Qingdao Daxue), 1999.
[11] Baanwal B.P., Das S.S. and Umme Farva, Progress in Crystal Growth and
Characterization of Materials, 2002, 45(1-2): 43.
[12] B. P. Baranwal , S. S. Das & Umme Farva, Indian J. of Chem., 2001, 40A: 893.
徐建中1,王春征1, 唐然肖1,2
(1.河北大学化学与环境科学学院 河北保定071002; 2.河北农业大学理学院 河北保定 071001)
摘要 本文采用六氯环三磷腈的乙氧基衍生物-六乙氧基环三磷腈作为配体,与无水CuCl2分别以不同的摩尔比进行络合,制备出新型络合物阻燃剂。用熔点测定仪、差热分析和红外等测试手段对其进行了表征。
关键词 六氯环三磷腈,络合物, 氯化铜,阻燃剂