| http://www.chemistrymag.org/cji/2006/08c072pe.htm | Dec.1,
2006 Vol.8 No.12 P.72 Copyright |
(1 School of Chemical Engineering / Shaanxi Key Laboratory of Physico-Inorganic Chemistry, Northwest University, Xi'an Shaanxi 710069; 2Conservation technology department, the Palace Museum, Beijing 100009; 3 Department of Chemistry / Shaanxi Key Laboratory of Physico-Inorganic Chemistry, Northwest University, Xi'an Shaanxi 710069, China) Abstract The title compound was synthesized by mixing 2-amino-4,6-dimethoxylpyrimidine, potassium thiocyanate and ethyl chloroformate in ethyl acetate. It was characterized by IR and element analysis. The specific heat capacity (Cp)was determined by a microcalorimetry apparatus Micro-DSC III, and Cp=0.331+3.463×10-3T (293.0≤T≤344.2K).The constant-volume combustion energy of the compound, (
Keywords synthesis; specific heat capacity; microcalorimetry; constant-volume combustion energy; standard molar enthalpy of formation Thioureas as a kind of highly active bactericide have been studied broadly for many years because they could prevent many crops from disease efficiently with little harm to crops and low toxicity to mammals [1]. Many research groups have made important contributions in this field [2-8].
The specific heat capacity is one of the essential thermodynamic data for substance, and importent to the relevant engineering technologic design of energies and materials. In this paper, the specific heat capacity and the constant-volume combustion energy of the title compound, 4-(4,6-dimethoxylpyrimidin-2-yl)-3-thio-allophonic acid ethyl ester, were determined by a microcalorimetry apparatus----Micro-DSC III and a precise rotating-bomb calorimeter respectively. Its standard molar enthalpy of combustion and standard molar enthalpy of formation were calculated on the basis of the constant-volume combustion energy of the compound. The final results would provide theoretical basis for enlarging their application field.
1. EXPERIMENTAL
1.1 Reagents and apparatus
2-Amino-4,6-dimethoxylpyrimidine was prepared in our laboratory according to the
literature[9] and all chemicals were of A.R. grade.
The C and H contents were measured by a Vario EL III CHNOS elemental analyzer made in Germany; IR spectra was recorded
with a Model EQUINOX 55 FTIR spectrophotometer (KBr pellet); the constant-volume
combustion energy of the compound was carried out by a RBC-type II precise rotating-bomb
calorimeter; the specific heat capacity of the compound was studied using a
microcalorimetry apparatus----Micro-DSC III (SETARAM, FRANCE); melting point of the
compound was measured with X-5 type digital melting-point apparatus.
1.2 Preparation of the title compound
The title compound used in this paper was
prepared according to the following method: 0.65g ethyl chloroformate was dropped slowly
into the solution of ethyl acetate that contained 0.388g potassium thiocyanate with
stirring under dry condition. After all of the methyl chloroformate was added, the
reaction was kept for 2h with stirring under reflux. Then,
the solution was filtrated while it was hot. 0.5g 2-amino-4,6-dimethoxyl pyrimidine was
added into the filtrate, and the reaction was
continued for 4h under the condition of reflux. The light yellow precipitate was obtained
after filtration, washed with distilled water and dried in a vacuum drier at 80℃. The product was refined with dimethylformylamine.
Anal. Calcd for C10H14N4O4S:
C 41.985, H 4.895, N 19.58; found: C 42.32, H 4.495, N 19.32. IR(KBr)
3516.40,1774.27,1168.02cm-1.
2. RESULTS AND DISCUSSION
2.1 Specific Heat Capacity of the compound
The enthalpies of solution (
Continuous heating model in Micro-DSC III apparatus was adopted in the experiment. It is convenient to measure the specific heat capacity of solid samples with this method. The specific heat capacity obtained by the experiments is a continuous variable temperature equation, and it is easy to deduce the specific heat capacity at a certain temperature according to the equation.
The schematic diagram of Cp determination is shown in Fig.1.Calculated formula is as equation (1).
where Cp (J·g-1·K-1) is the heat specific capacity, As and Ab (W) are the real-time heat flows of the sample and the blank respectively, ms (g) is the mass of sample, b (K·s-1) is the heating rate. The software program of apparatus can deal with the data and figure out the results automatically. The determination results with 0.28877g sample and 0.1K·min-1 heating rate was shown in Fig.2. The specific heat capacity of the title compound is obtained as Cp=0.331+3.463×10-3T (293.0≤T≤344.2).
Fig.1 Schematic diagram of continuous specific heat capacity
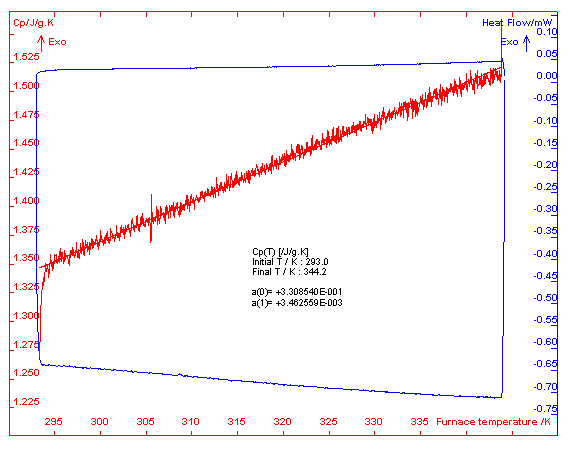
Fig. 2 Determination results of the specific heat capacity 2.2 Combustion energy of the compound
2.2.1 Experimental condition
The constant-volume combustion energy of the compound was determined by a precise rotating-bomb calorimeter (RBC-typeⅡ). The analytical methods and main experimental procedures were described previously [11].
The initial temperature was regulated to (25.0000 ± 0.0005) ℃, and the initial oxygen pressure was 2.5MPa. The correct value of the heat exchange was calculated according to Linio-Pyfengdelel-Wsava formula [12].
The calorimeter was calibrated with benzoic acid of 99.999% purity.(Chengdu Chemical Reagent Company), which has an isothermal heat of combustion of -2643 J·g-1 at 25℃. The calibrated experimental result was (17775.09±7.43) J·K-1 (Table 1), and the precision was 4.18×10-4. To determine the standard combustion energy of sulfur-containing compounds, the constant-volume combustion energy of thianthrene (mass fraction: 99%, recrystallized before using, Tokyo Kasei Kogyo Co. Ltd.) has been determined as being (-33507.76 ± 14.13) J·g-1 (Table 2), which suits well with the reported value as being(-33468 ± 4) J·g-1 . The precision and the accuracy were 4.22×10-4 and 1.19×10-3 , respectively.
The analysis methods of the final products (gas, liquid and solid) were the same as these in ref.[11].The analytical results of the final products indicated that the combustion reactions were complete. The calibrated experimental results were summarized in Table 1.
Table 1 Result for calibration of energy equivalent of the rotating-bomb calorimeter at 298.15K
NO. |
Mass of the compound |
Calibrated heat of combustion wire qc/J |
Calibrated heat of acid |
Calibrated DT/K |
Energy equivalent |
1 |
0.99702 |
10.35 |
24.78 |
1.4834 |
17790.45 |
2 |
0.78940 |
8.10 |
20.89 |
1.1746 |
17789.88 |
3 |
0.83060 |
12.60 |
20.43 |
1.2382 |
17758.93 |
4 |
0.96869 |
12.60 |
17.43 |
1.4418 |
17780.82 |
5 |
0.99485 |
12.60 |
20.80 |
1.4800 |
17798.18 |
6 |
1.12328 |
9.09 |
21.85 |
1.6735 |
17761.41 |
7 |
0.90036 |
9.28 |
21.67 |
1.3429 |
17745.97 |
Mean |
17775.09±7.43 |
2.2.2 Constant-volume combustion energy of the compound
The methods of determination and calculation of the constant-volume combustion energy for the compound are the same as for the calibration of the calorimeter with benzoic acid. The values are calculated by the following equation:
Where
Table 2 Experimental results for the combustion energies of the sample
Compound |
NO. |
Mass of the compound |
Calibrated heat of combustion wire |
Calibrated heat of acid |
Calibrated DT/K |
Combustion energy of sample |
1 |
1.15958 |
12.60 |
1563.51 |
1.1848 |
16802.48 |
|
2 |
1.06345 |
12.60 |
1433.89 |
1.0841 |
16760.06 |
|
3 |
1.10532 |
12.60 |
1490.35 |
1.1272 |
16767.21 |
|
4 |
1.13480 |
11.70 |
1530.13 |
1.1551 |
16734.38 |
|
5 |
1.08362 |
12.60 |
1461.09 |
1.1042 |
16752.70 |
|
6 |
1.12515 |
12.60 |
1510.91 |
1.1485 |
16784.59 |
|
Mean |
16766.90±9.81 |
|||||
Thianthrene |
1 |
0.48860 |
12.60 |
1383.69 |
0.9998 |
33514.62 |
2 |
0.49960 |
11.70 |
1384.41 |
1.0015 |
33558.98 |
|
3 |
0.49011 |
12.60 |
1387.90 |
1.0028 |
33511.58 |
|
4 |
0.48798 |
12.60 |
1381.96 |
0.9977 |
33484.26 |
|
5 |
0.48835 |
12.60 |
1382.99 |
0.9977 |
33456.78 |
|
6 |
0.48823 |
12.60 |
1382.65 |
0.9992 |
33520.31 |
|
Mean |
33507.76±14.13 |
2.2.3 Standard combustion enthalpy of the
compound
The standard molar combustion enthalpy of the compound,![]() , refers to the combustion enthalpy change of the following ideal
combustion reaction at 298.15K and 100kPa.
, refers to the combustion enthalpy change of the following ideal
combustion reaction at 298.15K and 100kPa.
![]() (3)
(3)
The standard molar combustion enthalpy of the compound is calculated by the following
equations:
![]() (4)
(4)
![]() (5)
(5)
Where ng is the total amount in mole of gases present as products or as
reactants, R=8.314J·K-1·mol-1,T=298.15K.The
result of the calculation of ![]() is (-4794.09
± 2.81)KJ·mol-1.
is (-4794.09
± 2.81)KJ·mol-1.
2.2.4 Standard molar enthalpy of formation for the compound
The standard molar enthalpy of formation of the compound,![]() , is calculated by Hess's law according to the
above thermochemical equation (3)
, is calculated by Hess's law according to the
above thermochemical equation (3)
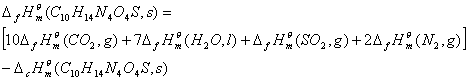
Where
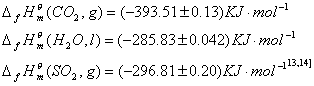
The result of the calculation of
The standard molar enthalpy of formation of the compound is negative, which indicates that the compound's stability is better, and it is easy for preparation, reserve and application. REFERENCES
[1] Wang D X., Bo Z S. New Pesticide Series.Wuhan: Central China Normal University Press, 1998
[2] Madan V K, Taneja A D. J. Indian. Chem. Soc. 1991, 68 (3):162
[3] Tang C C , Li Y, Chen B. Pesticide Chem.1988, 322
[4] Bowser A M. Tetrahedron Let. 2005, 46:2869
[5] Goodyear C L M. Bioorg. Med. Chem. 2003, 11:4189
[6] Harris J D, Eckles W E, Hepp A F, et al. Inorg. Chim. Acta. 2002, 338:99
[7] Sun X H, Gao R L, Liu Y F, et al. J. Chem. Engin.2003, 31(2):75
[8] REN Y H ,Song J R, Huang J, et al. Chin. J.Chem.2006, 24(2):219
[9] Kong F L, Wang L Q, Hu X H, et al .Huaxue Shijie.1991,(6):254
[10] Marthada, V. K. J. Res. Natl. Bur. Stand. 1980,85,467.
[11] Yang X W, Chen S P, Gao S L. J. Instrumentation Science &Technology, 2002,30(3):311
[12] Popov M M.Thermometry and Calorimetry. Moscow: Moscow University Publishing House, 1954:382
[13] Rederick D R.Experimental thermochemistry, Moscow: Interscience Publishers Ltd.1956:88
[14] Robert C W.CRC Handbook of Chemistry and Physics, Carson City: Chemical Rubber Publishing Company, 1977-1978, 55:D45 化合物4-(4,6-二甲氧基嘧啶-2-基)-3-硫代脲酸乙酯的热化学性质研究
任莹辉1,宋纪蓉1,2,徐抗震1,马海霞1,黄洁1,傅丁薇1,杨旭武3
(1西北大学化工学院/陕西省物理无机化学重点实验室,陕西 西安 710069; 2故宫博物院文保科技部 北京 100009 ;3西北大学化学系/ 陕西省物理无机化学重点实验室,陕西 西安 710069)
摘要 用2-氨基-4,6-二甲氧基嘧啶,硫氰酸钾和氯甲酸乙酯在乙酸乙酯中反应合成了标题化合物,通过红外光谱和元素分析法对化合物进行表征。用Micro-DSC Ⅲ微量量热仪测定了化合物的比热容Cp=0.331+3.463×10-3T (293.0≤T≤344.2K)。在298.15K下,用精密转动弹热量计测定出化合物的恒容燃烧能
关键词 合成;比热容;微量量热仪;恒容燃烧能;标准摩尔生成焓