| http://www.chemistrymag.org/cji/2006/08c075ne.htm | Dec.1,
2006 Vol.8 No.12 P.75 Copyright |
Li Zhiyun , Zhang Shuwen, Cheng Zhenhui , Wei
Baojun , Hao Pengpeng
(College of Chemistry and Environmental Science, Hebei University ,Baoding 071002, China)
Abstract N, N
'-Substituted bis(2, 5-dimethyl-3, 4-diethoxycarbonyl) pyrrole derivatives were synthesized via Paal-Knorr reaction of 3, 4–diethoxycarbonyl-2, 5 –hexanedione with diamine in the solvent of acetic acid. The structures of products were characterized by IR, 1H NMR spectra and elemental analysis.Keyword pyrrole derivatives, synthesis, Paal-Knorr reaction, diamine. 1 INTRODUCTION
Pyrrole and its derivatives are very important compounds as they occur in a large number of natural products and display a variety of physiological activities,[1] in particular, 1, 2, 3, 5-tetrasubstituted pyrrole derivatives have been proven to display antibacterial, [2] antiviral, [3] antiinflammatory[4] and antioxidant activities and to inhibit cytokine-mediated diseases. [5] Additionally, they have been found to show potent inhibiting platelet aggregation[6] and antihypertensive activities. [7] There are many methods to synthesize polysubstituted single pyrrole derivatives in recent years, however, few of polysubstituted bispyrrole compounds have been reported. The authors have synthesized a series of polysubstituted bispyrrole derivatives by Paal-Knorr reaction[8] [9] [10]. In this paper, ten N, N′- Substituted Bis(2, 5-dimethyl-3, 4- diethoxycarbonyl) pyrrole derivatives have been synthesized via Paal-Knorr reaction of 3, 4– diethoxycarbonyl-2, 5–hexanedione with diamine and acetic acid as solvent. The structures of products were characterized by IR, 1H NMR spectra and elemental analysis.
2 EXPERIMENT
2.1 Materials and instruments
The new compounds were characterized by data of 1H NMR, IR and element analyses and were described in the experimental section. IR spectra were recorded on a HITACHI 260-50 spectrometer(KBr). 1H NMR spectra were measured on an AVAVCE-400 spectrometer using TMS as internal standard and CDCl3 as solvent. Elemental analysis measured on a HERAEUS (CHN, Rapid) analyzer. M.p were measured on a XT-4 melting point instrument (thermometer uncorrected).
All of the reagents and solvents were AR grade. Absolute alcohol was dehydrated by distillation after sodium addition 3,4 –diethoxycarbonyl-2,5 –hexanedione was prepared by the method in literature. [11]
2.2 Preparation of N,N'-Substituted Bis(2,5-dimethyl-3,4-diethoxycarbonyl) pyrrole derivatives
To a flask with condensator were added 3,4 –diethoxycarbonyl-2,5 –hexanedione (0.516g, 2.00mmol) and diamine (1.00mmol) and acetic acid (15 mL) as solvent. The mixture was stirred and refluxed several hours. The procedure was monitored by TLC. (Scheme 1) After completion of the reactions, the mixture was cooled to room temperature and the products were purified by various methods.
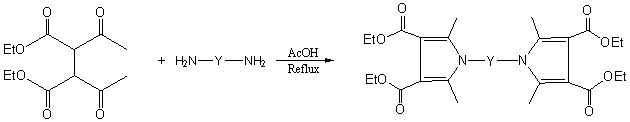
1 2a ~ 2j Scheme 1
2a Y = C6H4OC6H4 ; 2b Y = C6H4SO2C6H4 ; 2c Y = C6H4CH2C6H4; 2d Y = CH3C6H3C6H3CH3; 2e Y = C6H4C6H4; 2f Y = C6H4SO2NHC6H4; 2g Y = (CH2)2; 2h Y = (CH2)4; 2i Y = (CH2)6; 2j Y = (CH2)2 NH (CH2)2 NH (CH2)2. 2.2.1 Preparation of 4,4'-Bis (2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl) diphenyl ether (2a)
Diamine was 4,4'-bisamidobisphenylether. The product was extracted by absolute ether. The solvent was evaporated to give the crude product. The crude product was recrystallized by using 50% acetic acid as solvent to give white crystal 0.518g. The yield was 80.3%, m.p.138oC. 1H NMR (CDCl3, 400 MHz) d: 7.21 ( s, 8H, 8ArH ), 4.33 ( q, J = 7.2Hz, 8H, 4CH2 ), 2.20 ( s, 12H , 4Py-CH3。), 1.37 ( t, J = 7.2Hz, 12H, 4CH3). IR (KBr ) n: 3045, 1702, 1287, 1058, 1439, 1392, 1511, 1558, 847 cm-1; Anal.calcd for C36H40O9N2: C 67.06, H 6.25, N 4.35; found C 67.06, H 6.26, N 4.36.
2.2.2 Preparation of 4,4'-Bis ( 2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl ) diphenyl sulphone (2b)
Diamine was 4,4'-bisamidobisphenylsulphone. The product was extracted by absolute ether. The solvent was evaporated to give the crude product. The crude product was recrystallized by using anhydrous alcohol as solvent to give white crystal 0.567g. The yield was 81.9%. m.p. 174-176 oC. 1H NMR(CDCl3, 400 MHz) d: 8.19 ( d, J = 8.4Hz, 4H, 4ArH ), 7.42 ( d, J = 8.4Hz, 4H, 4ArH ), 4.32 ( q, J = 7.2Hz, 8H, 4CH2 ), 2.15 ( s, 12H, 4Py-CH3 ), 1.36 ( t, J = 7.2Hz, 12H, 4CH3 ). IR ( KBr ) n: 3035, 1700, 1283, 1022, 1431, 1397, 1500, 1558, 858 cm-1. Anal.calcd for C36H40O10N2S: C 62.42, H 5.82, N 4.05; found C 62.40, H 5.86, N 4.07.
2.2.3 Preparation of 4,4'-Bis ( 2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl ) diphenylmethane (2c)
Diamine was 4,4'-bisamidobisphenylmethane. The product was separated by Column chromatography [Petroleum ether and ethyl acetate (v/v: 2:1) as eluant] to give white crystal 0.465g. The yield was 72.4%, m.p. 147-148 oC. 1H NMR (CDCl3 400 MHz) d: 7.36 ( d, J = 8Hz, 4H, 4ArH ), 7.15 ( d, J = 8Hz, 4H, 4ArH ), 4.32 ( q, J = 7.2Hz, 8H, 4Et-CH2 ), 4.16 ( s, 2H, CH2 ), 2.17 ( s, 12H, 4Py-CH3 ), 1.36 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n: 3046, 1705, 1289, 1024, 1417, 1396, 1521, 1557, 833 cm-1. Anal. calcd for C37H42O8N2: C 69.14, H 6.59, N 4.36; found C 69.10, H 6.60, N 4.37.
2.2.4 Preparation of 3,3'-dimethyl-4,4'-Bis ( 2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl )diphenyl (2d)
Diamine was 3, 3'-bismethyl-4, 4'-bisamidobiphenyl. The water was added to the reactive mixture to give yellow precipitation. The precipitate was filtrated and recrystallized by using 75% acetic acid as solvent to give pale yellow crystal 0.519g. The yield was 79.1%, m.p.167-168oC. 1H NMR(CDCl3, 400 MHz) d: 7.21 ( d, J = 8Hz, 2H, 2ArH ), 7.12 ( m,, 4H, 4ArH ), 4.34 ( q, J = 7.2Hz, 8H, 4CH2 ), 2.15 ( s, 12H, 4Py-CH3 ), 2.09 ( s, 6H, 2Ar-CH3 ), 1.39 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n: 3030, 1712, 1292, 1031, 1435, 1392, 1500, 1559, 840, 773 cm-1. Anal.calcd for C38H44O8N2: C 69.49, H 6.75, N 4.27; found C 69.51, H 6.76, N 4.28.
2.2.5 Preparation of 4,4'-Bis(2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl) diphenyl (2e)
Diamine was 4,4'-bisamidobiphenyl. The water was added to the reactive mixture to give white precipitation. The precipitate was filtrated and recrystallized by using acetic acid and water as solvent to give pale white crystal 0.495g. The yield was 78.7%, m.p.200-202oC. 1H NMR(CDCl3, 400 MHz) d: 7.42 ( d, J = 8Hz, 4H, 4ArH ), 7.24 ( d, J = 8Hz, 4H, 4ArH ), 4.32 ( q, J = 7.2Hz, 8H, 4CH2 ), 2.16 ( s, 12H, 4Py-CH3 ), 1.36 ( t, J = 7.2Hz, 12H, 4CH3 ). IR ( KBr ) n 3035, 1710, 1295, 1025 , 1435, 1385, 1500, 1550, 840, 780 cm-1. Anal.calcd for C36H40O8N2: C 68.77, H 6.41, N 4.46; found C 68.76, H 6.43, N 4.43.
2.2.6 Preparation of 4,4'-Bis(2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl)-N-phenylbenzenesulfon- amide (2f)
Diamine was N-(p-amidophenyl)-p-amidobenzenesulfonamide. The water was added to the reactive mixture to give white precipitation. The precipitate was filtrated and recrystallized by using acetic acid as solvent to give white crystal 0.568g. The yield was 80.3%, m .p.102-104 oC. 1H NMR (CDCl3, 400 MHz) d: 8.02 ( d, J = 8.4Hz, 2H, 2ArH ), 7.33 ( d, J = 8.4Hz, 2H, 2ArH ), 7.29 ( t, J = 8.4Hz, 4H, 2ArH ), 4.32 ( q, J = 3.6Hz, 8H, 4CH2 ), 4.20 ( s, 1H, NH ), 2.12 ( s, 6H, 2Py-CH3 ) , 2.09 (s, 6H, 2py-CH3 ), 1.36 ( t, J = 3.6Hz, 12H, 4CH3 ). IR ( KBr ) n 3038, 1700 , 1291, 1029, 1438, 1394, 1521, 1559, 878 cm-1. Anal.calcd for C36H41O10N3S: C 61.09, H 5.84, N 5.94; found C 61.05, H 5.85, N 5.95.
2.2.7 Preparation of 1,2-Bis(2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl) ethane (2g)
Diamine was ethylenediamine. The absolute ether was added to the reactive mixture to give precipitation. The precipitate was filtrated to give white crystal 0.388g. The yield was 76.9%, m.p. 186-187 oC. 1H NMR (CDCl3, 400 MHz) d: 4.28 ( q, J = 7.2Hz, 8H, 4Et-CH2 ), 4.00 ( s, 4H, 2CH2 ), 2.28 ( s, 12H, 4Py- CH3 ), 1.33 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n 1703, 1315, 1056, 1438 , 1396 cm-1. Anal.calcd for C26H36O8N2: C 61.89, H 7.19, N 5.55; found C 61.86, H 7.20, N 5.56.
2.2.8 Preparation of 1,4-Bis(2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl) butane (2h)
Diamine was butanediamine. The absolute ether was added to the reactive mixture to give pale yellow precipitation. The precipitate was filtrated and recrystallized by using acetic acid as solvent to give white crystal 0.384g. The yield was 72.1%, m.p.172-174 oC. 1H NMR (CDCl3,400 MHz) d: 4.29 ( q, J = 7.2Hz, 8H, 4Et-CH2 ), 3.79 ( m, 4H, 2CH2 ), 2.39( s, 12H, 4Py-CH3 ), 1.67 ( m, 4H, 2CH2 ), 1.34 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n 1698, 1284, 1058, 1387, 1438 cm-1. Anal.calcd for C28H40O8N2: C 63.14, H 7.57, N 5.26; found C 63.30, H 7.59, N 5.27.
2.2.9 Preparation of 1,6-Bis(2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl) hexane (2i)
Diamine was hexanediamine. The water was added to the reactive mixture to give pale yellow precipitation. The precipitate was filtrated and dried to give pale yellow crystal 0.386g. The yield was 68.8%, m.p.156-158oC. 1H NMR (CDCl3, 400 MHz) d: 4.28 ( q, J = 6.8Hz, 8H, 4Et-CH2 ), 3.77 ( t, J = 7.6 Hz, 4H, 2CH2), 2.39 ( s, 12H, 4Py-CH3 ), 1.63 ( m, 4H, 2CH2 ), 1.38 (m, 4H, 2CH2), 1.35 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n 1697, 1281, 1059, 1418, 1392 cm-1. Anal.calcd for C30H44O8N2: C 64.26, H 7.91, N 5.00; found C 64.23, H 7.91, N 5.02.
2.2.10 Preparation of 2,2'-Bis[2,5-dimethyl-3,4-diethoxycarbonylpyrrol-1-yl)ethyl] ethylenediamine (2j)
Diamine was triethylenetetramine. The product was separated by Column chromatography (ethyl acetate as eluant) to give pale yellow oil matter that was recrystallized by using ethyl acetate and petroleum ether as solvent to give white crystal 0.404g. The yield was 68.4%, m.p.112-114 oC. 1H NMR (CDCl3,400 MHz) d: 4.29 ( q, J = 6.8Hz, 8H, 4Et-CH2 ), 4.00 ( s, 2H, 2NH ), 3.97 ( t, J = 7.2 Hz, 4H, 2CH2), 3.03 ( t, J = 7.2 Hz, 4H, 2CH2), 2.67 ( t, J = 7.0 Hz, 4H, 2CH2), 2.16 ( s, 12H, 4Py-CH3 ), 1.33 ( t, J = 7.2Hz, 12H, 4Et-CH3 ). IR ( KBr ) n 1700, 1297, 1059, 1439, 1403cm-1. Anal.calcd for C30H46O8N4: C 60.99, H 7.85, N 9.48; found C 60.97, H 7.84, N 9.46. 3 RESULTS AND DISCUSSION
The authors have synthesized ten N, N'-Substituted bis(2,5-dimethyl-3,4-diethoxycarbonyl) pyrrole derivatives in 70-80% yields conveniently.
In the literature, to synthesize pyrroles and pyrrole derivatives by the reaction of Paal-Knorr, the current solvent was the mixture of acetic acid and alcohol. In this paper, the time of reaction was shortened and the yield improved by using acetic acid as solvent. The reason was probably that the concentration of 3,4–dietho- xycarbonyl-2,5 –hexanedione was higher and the electrophilicity of carbonyl group was stronger in acetic acid.
To investigate study the effect of the materials ratio on products, the reaction ratio of the starting materials selected was 1:1. The result showed that primary products were bispyrrole derivatives and single pyrrole derivatives were lesser. 4 CONCLUSION
In this paper, ten pyrrole derivatives by the reaction of Paal-Knorr were synthesized. The products were all characterized by IR, 1H NMR spectra and elemental analysis. REFERENCES
[1] (a) Jones A, Bean G P. The Chemistry of Pyrroles. London: Academic Press, 1977; (b) Jones A. Pyrroles. New York: Wiley, 1990; (c) Paula M T, Ferreira, Hernani L S, et al. Tetrahedron Letters, 2002, 43(25): 4491.
[2] Daidone G, Maggio B, Schillari D. Pharmazie, 1990, 45: 441.
[3] (a) Almerico A M, Diana P, Barraja P, et al. Farmaco, 1998, 53: 33; (b) Almerico A M, Diana P, Barraja P, et al. Farmaco, 1997, 52: 667.
[4] Kaiser D G, Glenn E M. J. Pharm. Sci, 1972, 61: 1908.
[5] Lehuede J, Fauconneau B, Barrier L, et al. J. Med. Chem, 1999, 34: 991.
[6] Ohtsu A, Hoshina K, Jinba M, et al. Blood Vessel, 1979, 10: 5.
[7] Tilford C H, Hudak W J, Lewis R. J. Med. Chem, 1971, 14: 328; (b) Akhmedov S T, Sadygov N S, Ismailov V M, et al. Khim.Geterotsikl. Soedin, 1984, 10: 1428.
[8] Fan B G, Ming Y F, Fan M G. J. Org. Chem (Youji Huaxue ), 1994, 14: 599.
[9] Cheng Z H, Zhang S W, Li Z Y, et al. J. Org. Chem (Youji Huaxue ), 2006, 26 (1): 90.
[10] Zhang S W, Cheng Z H, Li Z Y, et al. CAIJ, 2005, 2 (7 ): 225.
[11] Han G D, Zhao S W, Li S W, et al. Chemical Handbook of Organic Preparation (Youji Zhibei Huaxue Shouce ). Beijing: Chemical Industry Press, 1980.
N,N′-取代二(2,5-二甲基-3,4-二乙氧羰基)吡咯衍生物的合成及表征
(河北大学化学与环境科学学院;中国河北保定 071002)
摘要 本文以3,4-二乙氧基羰基-2,5-己二酮和二元伯胺类化合物为原料通过 Paal-Knorr 反应,以乙酸为溶剂合成了N,N′-取代二 ( 2,5 -二甲基-3, 4-二乙氧羰基)吡咯衍生物,并通过红外光谱、核磁共振氢谱及元素分析对化合物进行了表征.
关键词 吡咯衍生物, 合成, Paal-Knorr反应, 胺类化合物