http://www.chemistrymag.org/cji/2007/091001pe.htm |
Jan. 2,
2007 Vol.9 No.1 P.1 Copyright |
Acylating activity of hydroxyl groups in 2' - hydroxyethyl 3a,7a,12a-trihydroxy - 5b - cholan - 24-ate
Hu
Xiangzheng 1 , Liu Anjun2, Wang Lixia2, Liu Yanhong1,
Ni Liqin1
(1.College of Science, Tianjin University of Science and Technology, Tianjin, 300457,
China; 2. College of Food Science and Biotechnology, Tianjin University of Science and
Technology, Tianjin, 300457, China)
Received Nov. 21, 2006.
Abstract
Acylating activity of hydroxyl groups in 2' - hydroxyethyl 3a , 7a , 12a - trihydroxy - 5b - cholan - 24 - ate has been determined by the use of methacryloyl chloride, methacryloyl anhydride and methacrylic acid as the acylating agents. The results show: that the primary hydroxyl group has the highest reactivity in all four hydroxyl groups. The relative reactivity of secondary hydroxyl groups on steroids skeleton is various with three kinds of acylating agents.Ketword 2'- hydroxyethyl 3a , 7a , 12a - trihydroxy - 5b-cholan- 24- ate; acylating; reactivity
1 INTRODUCTION
Bile acids are natural compounds that are synthesized from cholesterol in the liver,
as sodium salts of N-acyl derivatives of glycine and taurine and play important
physiological roles in the body. They are stored in the gallbladder and released during
meals for the digestion of fats and lipids in food and reabsorbed in the intestine to
complete the enterohepatic circulation with very little loss. All bile acids possess a
steroid skeleton, a carboxylic acid group and different hydroxyl groups, chemical
modifications can be made easily on these groups. They are all amphiphilicity molecules
and can form micelles and other supramolecular structures in stepwise manner [1, 2,
3]. Compounds derived from bile acids are expected to be safe and nontoxic when used
in biomedical and pharmaceutical fields. In recent years,
bile acids have been used in the development of new polymeric materials used in drug
delivery system[4,5], asymmetric synthesis[6,7], molecular
recognition [8,9] and polymeric biomaterials[10-12].
3a , 7a , 12a - trihydroxy - 5b - cholan - 24 - oic acid, or cholic acid , one of the most
commonly occurring bile acids, consists of a steroid skeleton, one carboxylic group and
three hydroxyl groups, which exhibit very different reactivity depending on their location
on the steroid skeleton [13]. Literature work reported that the reactivity of
the three hydroxyl groups on steroid skeleton follows the order C3 OH >> C7 OH >
C12 OH for esterification[1,2,14]. In the previous work, the authors used
methacryloyl chloride as acylating agent to modify cholic acid derivatives and found the
reactivity of secondary hydroxyl groups on steroids backone follows the order C3 OH >
C12 OH > C7 OH [15]. In this paper, the authors used methacryloyl chloride,
methacryloyl anhydride and methacrylic acid as the acylating agents to study acylation
activity of hydroxyl groups in 2' - hydroxyethyl 3a , 7a
, 12a - trihydroxy - 5b - cholan - 24- ate (1) (Figure 1).
2 EXPERIMENTAL
2.1 Materials and instruments
All the chemicals were of reagent grade and used without further purification. All the
anhydrous solvents were freshly purified by standard techniques. IR spectra were record on
FTS135-FTIR spectrophotometer (Bio-Rad Instruments) with KBr pellets. NMR spectra were
recorded at 23°C on a Bruker AMX- 400 operating at 400.4 MHz for 1H in CDCl3
and TMS were used as the solvent and internal standard. Elemental analyses were carried
out on Foss Heraeus.
The chemical structures of 1 and its methacrylate derivatives (2
~ 6) are shown in Figure 1.
2.2 2'-Hydroxyethyl 3a ,7a ,12a
-trihydroxy-5b -cholan-24-ate (1)
The product 1 was synthesized by using a procedure of the one published in the
literature [15]. m.p. 135-136°C; IR ![]() /cm-1: 3351.7, 2935.8, 2866.5, 1733.7, 1464.0,
1375.5, 1176.5, 1078.6, 980.9, 913.4; 1H NMR (400MHz, CDCl3), d :
0.69 (s, 3H, 18-H3), 0.89 (s, 3H, 19-H3), 1.00 (d, J = 6.0Hz, 3H,
21-H3), 3.43 (m, 1H, 3a -H), 3.82 (m, 3H, 7a -H, COOCH2CH2OH),
3.97 (s, 12a -H), 4.21 (t, 2H, COOCH2CH2OH); Anal. Calcd for
C26H44O6 (452.63): C 68.99 %, H 9.80 %; found: C 68.84 %,
H 9.87 %.
/cm-1: 3351.7, 2935.8, 2866.5, 1733.7, 1464.0,
1375.5, 1176.5, 1078.6, 980.9, 913.4; 1H NMR (400MHz, CDCl3), d :
0.69 (s, 3H, 18-H3), 0.89 (s, 3H, 19-H3), 1.00 (d, J = 6.0Hz, 3H,
21-H3), 3.43 (m, 1H, 3a -H), 3.82 (m, 3H, 7a -H, COOCH2CH2OH),
3.97 (s, 12a -H), 4.21 (t, 2H, COOCH2CH2OH); Anal. Calcd for
C26H44O6 (452.63): C 68.99 %, H 9.80 %; found: C 68.84 %,
H 9.87 %.
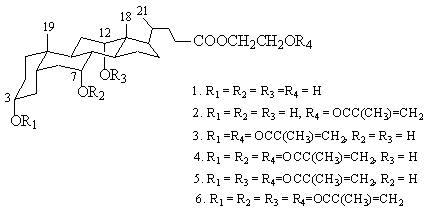
Figure 1 The chemical structures of 2¢
- hydroxyethyl 3a , 7a , 12a - trihydroxy - 5b - 24 - ate and its methacrylate derivatives
2.3 2'-Methacryloyloxyethyl 3a ,7a ,12a
-trihydroxy-5b
-cholan-24-ate(2)
The product 2 was synthesized by the use of a procedure of the one published in
the literature[15]. IR ![]() /cm-1:
3492.1, 2939.8, 2868.6, 1717.9, 1637.4, 1452.8, 1379.6, 1268.7, 1168.8, 940.2; 1H
NMR (400MHz, CDCl3), d : 0.67 (s, 3H, 18-H), 0.88 (s, 3H, 19-H), 0.97 (d, J =
6.0Hz, 3H, 21-H), 1.94 (s, 3H, C(CH3)=CH2), 3.43 (m, 1H,
3-H), 3.84 (s, 1H, 7-H), 3.96 (s, 1H, 12-H), 4.33 (s, 4H, COOCH2CH2COO),5.60 (s ,1H, =CH), 6.13 (s ,1H, =CH); Anal. Calcd for
C30H48O7 (520.71): C69.20 %, H9.29 %; found: 69.02 %, H
9.39 %.
/cm-1:
3492.1, 2939.8, 2868.6, 1717.9, 1637.4, 1452.8, 1379.6, 1268.7, 1168.8, 940.2; 1H
NMR (400MHz, CDCl3), d : 0.67 (s, 3H, 18-H), 0.88 (s, 3H, 19-H), 0.97 (d, J =
6.0Hz, 3H, 21-H), 1.94 (s, 3H, C(CH3)=CH2), 3.43 (m, 1H,
3-H), 3.84 (s, 1H, 7-H), 3.96 (s, 1H, 12-H), 4.33 (s, 4H, COOCH2CH2COO),5.60 (s ,1H, =CH), 6.13 (s ,1H, =CH); Anal. Calcd for
C30H48O7 (520.71): C69.20 %, H9.29 %; found: 69.02 %, H
9.39 %.
2.4 2'-Methacryloyloxyethyl 3a -methacryloyloxy-7a ,12a
-dihydroxy-5b
-cholan-24-ate(3)
The product 3 was synthesized by the use of a procedure of the one published in
the literature[15]. IR ![]() /cm-1:
3508.0, 2942.8, 2871.2, 1717.4, 1635.9, 1452.3, 1377.3, 1296.7, 1072.9, 940.1; 1H
NMR (400MHz, CDCl3), d : 0.69 (s, 3H, 18-H3), 0. 91 (s, 3H, 19-H3),
0.98 (d, J = 6.4Hz, 3H, 21-H3), 1.91 (s, 3H, C(CH3)=CH2),
1.94 (s, 3H, C(CH3)=CH2), 3.86 (s, 1H, 7a -H), 3.99 (s, 1H,2a -H), 4.33 (s, 4H, COOCH2CH2OCO),
4.63 (m, 1H, 3a -H), 5.50 (s, 1H,=CH), 5.60
(s, 1H, =CH), 6.07 (s, 1H, =CH), 6.13
(s, 1H, =CH); Anal. Calcd for C34H52O8 (588.78): C
69.36 %, H 8.90 %; found: C 69.15 %, H 9.06 %.
/cm-1:
3508.0, 2942.8, 2871.2, 1717.4, 1635.9, 1452.3, 1377.3, 1296.7, 1072.9, 940.1; 1H
NMR (400MHz, CDCl3), d : 0.69 (s, 3H, 18-H3), 0. 91 (s, 3H, 19-H3),
0.98 (d, J = 6.4Hz, 3H, 21-H3), 1.91 (s, 3H, C(CH3)=CH2),
1.94 (s, 3H, C(CH3)=CH2), 3.86 (s, 1H, 7a -H), 3.99 (s, 1H,2a -H), 4.33 (s, 4H, COOCH2CH2OCO),
4.63 (m, 1H, 3a -H), 5.50 (s, 1H,=CH), 5.60
(s, 1H, =CH), 6.07 (s, 1H, =CH), 6.13
(s, 1H, =CH); Anal. Calcd for C34H52O8 (588.78): C
69.36 %, H 8.90 %; found: C 69.15 %, H 9.06 %.
2.5 2'-Methacryloyloxyethyl 3a ,7a
-dimethacryloyloxy-12a
-hydroxy-5b
-cholan-24-ate(4)
The product 4 was synthesized by the use of a modified procedure of the one published
in the literature[14]. 1(2.00
g,4.42 mmol), DCC (4.56g, 22.1mmol) and DMAP (0.27 g
,2.21 mmol) were dissolved in DCM (40 mL).Thereafter, methacrylic acid(1.90g,22.1 mmol,dissolved in10 mL DCM) was added dropwise and the reaction allowed
to proceed overnight with stirring. The solid was filtered off and the solution washed
successively with brine, 5% acetic acid, 5 % aqueous Na2CO3, and
brine. After drying the organic layer over anhydrous Na2SO4, the
solvent was evaporated under reduced pressure, and the crude product was purified by
column chromatography (silica gel, ethyl acetate/petroleum = 3/7 as the eluent).
Yield:1.79 g (62 %). IR ![]() /cm-1:
2943.5, 1711.4, 1637.2, 1450.8, 1295.3, 1159.1 cm-1 ; 1H NMR (400MHz, CDCl3),
d : 0.75 (s, 3H, 18-H3), 0.83 (s, 3H, 19-H3), 0.89 (d, J = 6.4Hz,
3H, 21-H3), 1.89 (s, 3H, C(CH3)=CH2), 1.93 (s, 3H, C(CH3)=CH2),
1.98 (s, 3H, C(CH3)=CH2), 3.98 (s, 1H,12a -H), 4.31 (s, 4H, COOCH2CH2OCO), 4.60 (m,
1H, 3a -H), 5.02 (s, 1H, 7a -H), 5.50 (s, 1H, =CH), 5.56 (s, 1H, =CH), 5.58 (s, 1H, =CH),
6.03 (s, 1H,=CH), 6.11 (s, 1H, =CH), 6.13 (s,1H, =CH);Anal. Calcd for C34H52O8 (656.86):
C 69.49%, H 8.59%; found: C 69.73%, H 8.42%。
/cm-1:
2943.5, 1711.4, 1637.2, 1450.8, 1295.3, 1159.1 cm-1 ; 1H NMR (400MHz, CDCl3),
d : 0.75 (s, 3H, 18-H3), 0.83 (s, 3H, 19-H3), 0.89 (d, J = 6.4Hz,
3H, 21-H3), 1.89 (s, 3H, C(CH3)=CH2), 1.93 (s, 3H, C(CH3)=CH2),
1.98 (s, 3H, C(CH3)=CH2), 3.98 (s, 1H,12a -H), 4.31 (s, 4H, COOCH2CH2OCO), 4.60 (m,
1H, 3a -H), 5.02 (s, 1H, 7a -H), 5.50 (s, 1H, =CH), 5.56 (s, 1H, =CH), 5.58 (s, 1H, =CH),
6.03 (s, 1H,=CH), 6.11 (s, 1H, =CH), 6.13 (s,1H, =CH);Anal. Calcd for C34H52O8 (656.86):
C 69.49%, H 8.59%; found: C 69.73%, H 8.42%。
2.6 2'-Methacryloyloxyethyl 3a ,12a -dimethacryloyloxy-7a -hydroxy-5b -cholan-24-ate(5)
Starting with 1 (1.00 g, 2.21 mmol), DMAP (0.08 g, 0.65mmol) , Et3N
(0.9 g, 8.9 mmol) and methacryloyl chloride (0.93 g, 8.9 mmol), the synthesis took place
as described for 3. Yield: 0.62 g (43 %); IR ![]() /cm-1: 2943.2, 2871.5, 1711.9, 1636.4, 1450.1,
1320.3, 1294.9, 1158.6, 938.6; 1H NMR (400MHz, CDCl3), d : 0.75 (s,
3H, 18-H3), 0.81 (d, J = 6.4Hz, 3H, 21-H3), 0.89 (s, 3H, 19-H3),
1.89 (s, 3H, C(CH3)=CH2), 1.93 (s, 3H, C(CH3)=CH2),
1.98 (s, 3H, C(CH3)=CH2), 3.87 (s, 1H,7a -H), 4.31 (s, 4H, COOCH2CH2OCO),
4.59 (m, 1H, 3a -H), 5.16 (s, 1H, 12a -H), 5.50 (s, 1H, =CH), 5.56 (s, 1H, =CH),
5.58 (s, 1H, =CH), 6.03 (s, 1H,=CH), 6.11 (s, 1H, =CH), 6.13 (s,1H,
=CH); Anal. Calcd for C34H52O8 (656.86): C 69.49
%, H 8.59 %; found: C 69.77 %, H 8.51 %.
/cm-1: 2943.2, 2871.5, 1711.9, 1636.4, 1450.1,
1320.3, 1294.9, 1158.6, 938.6; 1H NMR (400MHz, CDCl3), d : 0.75 (s,
3H, 18-H3), 0.81 (d, J = 6.4Hz, 3H, 21-H3), 0.89 (s, 3H, 19-H3),
1.89 (s, 3H, C(CH3)=CH2), 1.93 (s, 3H, C(CH3)=CH2),
1.98 (s, 3H, C(CH3)=CH2), 3.87 (s, 1H,7a -H), 4.31 (s, 4H, COOCH2CH2OCO),
4.59 (m, 1H, 3a -H), 5.16 (s, 1H, 12a -H), 5.50 (s, 1H, =CH), 5.56 (s, 1H, =CH),
5.58 (s, 1H, =CH), 6.03 (s, 1H,=CH), 6.11 (s, 1H, =CH), 6.13 (s,1H,
=CH); Anal. Calcd for C34H52O8 (656.86): C 69.49
%, H 8.59 %; found: C 69.77 %, H 8.51 %.
2.7 2'-Methacryloyloxyethyl 3a ,7a
,12a -trimethacryloyloxy -5b -cholan-24-ate(6)
Starting with 1 (1.00 g, 2.21 mmol), Et3N (2.24 g, 22.1 mmol) and
methacryloyl chloride (1.84 g, 17.68 mmol), The synthesis was carried out as described for
3. The crude product was purified by column chromatography (silica gel, ethyl
acetate/petroleum = 1/5 as the eluent). Yield: 1.27 g (79 %); IR ![]() /cm-1: 2954.2, 2871.5, 1711.7, 1637.2, 1450.3,
1317.6, 1294.0, 1149.4, 936.5, 812.8; 1H NMR (400MHz, CDCl3), d :
0.75 (s, 3H, 18-H3), 0.81 (d, J = 6.0Hz, 3H, 21-H3), 0.94 (s, 3H,
19-H3), 1.88 (s, 3H, C(CH3)=CH2), 1.92 (s, 3H, C(CH3)=CH2),
1.95 (s, 3H, C(CH3)=CH2), 1.98 (s, 3H, C(CH3)=CH2),
4.30 (s, 4H, COOCH2CH2OCO), 4.63 (m, 1H, 3a -H), 5.01
(s, 1H, 7a -H), 5.20 (s, 1H, 12a -H), 5.49 (s, 1H,
=CH), 5.54 (s, 1H, =CH), 5.56 (s, 1H, =CH), 5.57 (s, 1H, =CH),
5.99 (s, 1H, =CH), 6.10 (s, 2H, =CH×2), 6.12 (s, 1H, =CH); Anal.
Calcd for C42H60O10 (724.93): C 69.59 %, H 8.34 %; found:
C 69.68 %, H 8.52 %.
/cm-1: 2954.2, 2871.5, 1711.7, 1637.2, 1450.3,
1317.6, 1294.0, 1149.4, 936.5, 812.8; 1H NMR (400MHz, CDCl3), d :
0.75 (s, 3H, 18-H3), 0.81 (d, J = 6.0Hz, 3H, 21-H3), 0.94 (s, 3H,
19-H3), 1.88 (s, 3H, C(CH3)=CH2), 1.92 (s, 3H, C(CH3)=CH2),
1.95 (s, 3H, C(CH3)=CH2), 1.98 (s, 3H, C(CH3)=CH2),
4.30 (s, 4H, COOCH2CH2OCO), 4.63 (m, 1H, 3a -H), 5.01
(s, 1H, 7a -H), 5.20 (s, 1H, 12a -H), 5.49 (s, 1H,
=CH), 5.54 (s, 1H, =CH), 5.56 (s, 1H, =CH), 5.57 (s, 1H, =CH),
5.99 (s, 1H, =CH), 6.10 (s, 2H, =CH×2), 6.12 (s, 1H, =CH); Anal.
Calcd for C42H60O10 (724.93): C 69.59 %, H 8.34 %; found:
C 69.68 %, H 8.52 %.
3 RESULTS AND DISCUSSION
3.1 Synthesis of 2' - hydroxyethyl 3a ,7a ,12a - trihydroxy - 5b - cholan - 24 - ate derivatives
1 ~ 6 were synthesized from cholic acid. Methacryloyl chloride, methacryloyl
anhydride and methacrylic acid were used as the acylating agents in order to determine the
hydroxyl groups reactivity of 1. The results show: When methacrylic acid was used
with DCC (dehydrating agent) and DMAP (catalyst), the products obtained were 2, 3,
4 and 6 respectively, indicating that the order of reactivity for four
hydroxyl groups in 1 is 1° OH > C3 OH > C7 OH> C12 OH. When methacryloyl
chloride was used, compounds 2, 3, 5 and 6 obtained. Thin-lay chromatography was used to follow the tracks of the
reaction, which shown 1° OH 、C3 OH、C12 OH and C7 OH reacted with methacryloyl chloride on this order
in the course of reaction with the increase of methacryloyl chloride. Therefore, the
reactivity of the OH groups follow the order of 1° > C3 > C12 > C7 for
methacryloyl chloride. To this reaction, we found it is difficult to make it ceasing on 1°
OH by controlling the addition of methacryloyl chloride. When
the input of methacryloyl chloride is enough, C3 OH reacted with it before the reaction on
1° OH finished.
All five compounds 2, 3, 4, 5 and 6
can be obtained by the use of methacryloyl anhydride as acylating agent. For the reaction,
2, 3 and 6 can be obtained as the only product by controlling the
adding of methacryloyl anhydride, while 4 and 5 are always obtained at the
same time. The addition of DMAP accelerates the reaction. The higher temperature also
shortens the reaction time and seems to favor the formation of 5. This indicating
the reactivity for four hydroxyl groups in 1 is 1° > C3 > C7 » C12
when methacryloyl anhydride was used.
3.2 Reactivity of hydroxyl groups in 2' - hydroxyethyl 3a , 7a , 12a - trihydroxy
- 5b - cholan - 24- ate
Cholic acid belong to coprostane steroids, all the flat rings of skeleton are on the same
plane, where the cis connection ring A and ring B imparts a curvature to the steroids
skeleton, making possible the formation of a cavity. C18 - CH3, C19 - CH3
and C21 - CH3 are on completely hydrophobic b face, C3 OH, C7 OH and C12 OH are
directed to a face, forming the hydrophilic part together with the carboxylic side chain.
The three secondary hydroxyl groups of 1 are on the same face of the steroid
skeleton, C3 OH is at an equatorial position therefore less sterically hindered and has a
higher reactivity than C7 OH and C12 OH [16]. C12
OH should have a lower reactivity than that of C7 OH because the distance between this
group and 21-CH3 is shorter and thus more sterically hindered.
The acylation easily occurs on the hydroxyl groups of 1 when
methacryloyl chloride is used as the acylating agent, Et3N as absorption agent
of HCl. In the solution, methacryloyl cation can be released smoothly from methacryloyl
chloride and it attacks the hydroxyl groups of 1, the reaction occurred. Compared
to the secondary hydroxyl groups on skeleton, the primary hydroxyl has less sterically
hindered, so a higher reactivity is. C3 OH has less sterically hindered than C7 OH and C12
OH, when methacryloyl chloride is used, the reaction occurs on it before C7 OH and C12 OH. Once C3 OH is acylated, the reactivity of C12 OH is enhanced since
the methacryloyl group is electron-withdrawing [17], while steric hindrance of
the same group for C7-OH is more significant. Therefore, the reactivity of the hydroxy
groups of 1 follows the order of 1° > C3 > C12 > C7.
When methacrylic acid is used as the acylating reagent, the
methacryloyl cation can not be created in the solution directly, so the acylating reaction
do not occur directly. The complex between methacrylic acid
and DMAP is formed when the DMAP presented, the acylating reactivity of methacrylic acid
is improved, so the reaction can occur. To four hydroxyl groups of 1, the primary
hydroxyl group has less sterically hindrance and the highest reactivity. For the three
second hydroxyl groups, the sterically hindered follows the order C3 < C7 < C12,
therefore the reactivity follows the order C3 OH > C7 OH > C12 OH.
Methacryloyl cation can be created from methacryloyl anhydride in the
solution and react with the hydroxyl groups of 1. The primary hydroxyl group has
the least sterically hinderence, so 2 can be produced at first. The producing rate
of methacryloyl cation from methacryloyl anhydride is not as quick as from methacryloyl
chloride, so the reaction can take place only on primary hydroxy group by controlling the
adding of methacryloyl anhydride. For the three second hydroxyl groups, the steric
hindrance follows the order C3 > C7 > C12, therefore, after primary hydroxyl is
acylated, the reaction goes to on C3 OH and 3 presented. Continue to increase the
input of methacryloyl anhydride, C7 OH is acylated and 4 is created. After the
methacryloyl group is bound on C3 OH, the reactivity of C12 OH is enhanced, while steric
hindrance of the same group for C7-OH is more significant, which leads the reactivity
difference between C7 OH and C12 OH little. So 4 and 5 can be produced at
the same time in this system.
ACKNOWLEDGEMENTS The authors thank Tianjin University of Science and Technology for the financial support of this work.
REFERENCES
[1] Li Y, Dias J R. Chem. Rev, 1997, 97 (1): 283.
[2] Zhu X X, Nichifor M. Acc. Chem. Res, 2002, 35 (7): 539.
[3] Denike J K, Moskova M, Zhu X X. Chem. Phys. Lipids,1995, 77: 261.
[4] Ghedini N, Ferruti P. Synth. Commun., 1983, 13 (8): 701.
[5] Noll O, Ritter H. Macromol. Rapid. Commun.,1996,
17 (9): 553.
[6] Maitra U, Mathivanan P. J. Chem. Soc: Chem. Commun., 1993, (19): 1469.
[7] Maitra U, Bag B G. J. Org. Chem., 1992, 57 (25): 6979.
[8] Bonar-Law R P, Davis A P. Tetrahedron, 1993, 49 (43): 9829.
[9] Maitra U, Bag B G. J. Org. Chem, 1994, 59 (20) :
6114.
[10] Zhang Y H, Akram M, Liu H Y, et al. Macromol. Chem. Phys., 1998,199 (7): 1399.
[11] Benrebouh A, Zhang Y H, Zhu X X. Macromol. Rapid. Commun.,2000, 21: 685.
[12] Benrebouh A, Avoce D, Zhu X X. Polymer, 2001, 42 (9): 4031.
[13] Baker J F, Blickenstaff R T. J. Org. Chem., 1975, 40 (11): 1579.
[14] Sellergren B, Wieschemeyer J, Boos K-S, et al. Chem. Mater., 1998, 10 (12): 4037.
[15] Hu X Z, Zhang Z, Zhang X, et al. Steroids, 2005, 70: 531.
[16] Brown H C. J. Am. Chem. Soc., 1938, 60: 1325.
[17] Blickenstaff R T, Sophasan K. Tetrahedron, 1972, 28: 1945.
2'-羟基胆酸乙酯羟基酰化反应活性研究
胡祥正, 刘安军2,王丽霞2, 刘雁红1,倪丽琴1
(1.天津科技大学理学院, 天津 300457; 2.天津科技大学食品科学与生物技术学院, 天津 300457)
摘要 应用甲基丙烯酰氯、甲基丙烯酸酐和甲基丙烯酸作酰化试剂考察2'- 羟基胆酸乙酯分子中羟基的酰化反应活性。结果显示:与三种酰化试剂反应,2'- 羟基胆酸乙酯分子四个羟基中的伯羟基反应活性都最高,甾体骨架上三个仲羟基的相对反应活性顺序因酰化剂活性差别而不同。
关键词 2'- 羟基胆酸乙酯;酰化;反应活性