http://www.chemistrymag.org/cji/2007/092006ne.htm |
Feb. 2,
2007 Vol.9 No.2 P.6 Copyright |
Gao Jungang, Liu Zhanli, Liu Pengyan,
Jiang Ning
(College of Chemistry and Environmental
Science, Baoding 071002,China)
Received Dec. 10, 2006.
Abstract Molecular imprinted polymers (MIPs) for ciprofloxacin were synthesized using methacrylic acid as a functional monomer and trimethylolpropane trimethacrylate(TRIM) as a cross-linker. The binding property and recognition selectivity were studied by equilibrium binding test. The MIP showed high selectivity and specificity to ciprofloxacin, separation factor a=2.03. Scatachard analysis indicated that two kinds of sites were formed in MIP for ciprofloxacin and the imprinting mechanism was discussed.Keywords molecularly imprinted polymer;Ciprofloxacin;Molecular recognition
1. INTRODUCTION
Molecularly imprinted polymers (MIPs) are produced by forming a polymer around a
molecule that is used as a template (print molecule). After removal of the print molecule,
the polymer can be used as a selective binding medium for the print molecule or
structurally related compounds. MIPs have been made selective for a large variety of
compounds and have been widely applied in analytical separation science [1],
membrane separation, stationary phase in chromatography [2], biological sensors
[3], simulating enzyme-catalyzed reactions [4].
Quinolones are antibiotics used widely to prevent and treat a large
variety of infectious diseases in human and veterinary medicine [5]. It is well
known that residues of such antibiotics may persist in edible animal tissues, which makes
quinolones potentially hazardous to human health. Most of the analytical methods
established thus far for quinolones are based on spectrophotometry and liquid
chromatography (LC) [6]. Quinolones imprinted polymers have been prepared [7,8].
Ester Caro et al. have applied enrofloxacin imprinted polymer to the solid-phase
extraction of fluorinated quinolones from urine and tissue samples [9].
Ciprofloxacin (CPFX), levofloxacin (L-FLX) and norfloxacin (NFLX) are
one group of quinolones antibiotics. In this paper, MIP with ciprofloxacin as template
molecule was prepared by bulk polymerization and was used as a selective sorbent in
equilibrium binding studies.
CPFX
L-FLX
NFLX
Scheme 1
2. EXPERIMENTAL
2.1. Reagents and standards
2.2. Instrumentation
The morphology structure of MIP was observed using a scanning electron microscope (SEM, KYKY-1000B, China).
The chromatographic system (LC-10Avp) was used in the analytical process, and the chromatographic separation was achieved on a chromatographic column (VP-ODS, 250mm×4.6mm, 5mm). Detection was performed with a SPD-M10Avp diode array detector and they are all from Shimadzu Co of Japan. The mobile phase consisted of a mixture of 0.02 M phosphate buffers-triethylamine, pH 2.80 and methanol (72:28, v/v) and used at a 1 mL /min flow-rate. The column temperature was 40oC.
2.3. Preparation of the imprinted polymer
A CPFX-imprinted polymer was prepared with CPFX as the template (0.331 g, 1mmol) and Toluene as the porogen (6.0 mL). CPFX was dissolved in the Toluene in a 25 mL thick-walled glass tube. Then, MAA (0.517g, 6.0 mmol), TRIM (6.76 g, 20.0 mmol) and AIBN (0.08 g, 0.51 mmol) were added and sparged with oxygen-free nitrogen gas for 5 min. The tube was sealed under nitrogen and heated in a water bath set at 60oC for 24h. After this period, the bulk cross-linked polymer was crushed in a mortar, and the template was extracted with MeOH-HAc(9:1, v/v) in a Soxhlet apparatus for 12h.
2.4. Binding experiments
The sized and washed polymer particles (20.0 mg) were placed in a 50 mL conical flask and mixed with 20 mL of a known concentration of selected substrate in water. The conical flask was oscillated in a constant temperature bath oscillator at 25oC for 16h until the equilibrium was attained. The concentration of free substrate in the solution was determined using a UV-VIS-spectrophotometer.
20.0mg MIP were incubated 16h and at room temperature with 200mg/L ciprofloxacin or levofloxacin in 20mL water. A rocking table ensured gentle mixing. The MIP were then separated by filter. The concentration of free substrate in the supernatant was measured using liquid chromatography at 281 nm.
3. RESULT AND DISCUSSION
3.1. SEM and TG analysis of MIPs
Fig.1 shows the SEM photographs of MIP surface. As can be seen from Fig.1, there are
many holes on the surface of MIP, these are holes to come out and go in for imprinted
molecules.

Fig.1 SEM photographs of MIP
3.2. Binding kinetics and binding property of
MIP
The Binding capacities of MIP and non-imprinted polymers (NIP) were measured by the
procedure, Adsorption capacity–adsorption time curve
of MIP showed that MIP of CPFX prepared based on bulk polymerization, reached max binding
capacities after 5h at 25oC. It showed that the adsorption rate was more
quickly. The later binding studies were kept for 16 h for ensuring reaching equilibrium.
The results are shown in Table 1. As seen in Table 1, this MIP exhibits a higher binding capacity for CPFX than that all of the other tested substrates. However, NIP exhibits similarly low values of Q (amount of adsorbed substrate) for all of the substrates. The results indicate that the imprinting method creates a microenvironment based on shape selection and position of functional groups for recognizing the CPFX template molecule. The selectivity test also gave illuminated aspects of the molecular recognition mechanism.
Table
.1 The binding capacities of MIP for substratesSubstrate |
CPFX |
L-FLX |
NOR |
QMIP ( mmol/L) |
141.88 |
62.07 |
46.32 |
QNIP ( mmol/L) |
62.05 |
36.99 |
38.84 |
Polymers: 20mg, initial concentration of substrate=1.0mmol/L, V=20.0mL, T=25oC,
3.3. Selectively binding of MIP
The molecular recognition selectivity of MIP and Non-MIP was
evaluated by chromatographic analysis of the concentration of the mixed levofloxacin and
ciprofloxacin. The procedure of determined selectivity under equilibrium conditions was
the same as that the determination of adsorption capacity. The difference was only the
solution used for selectivity studies contained one competing substrate along with CPFX.
Concentrations of free sorbates were detected by their absorbance at 281 nm using
reverse-phase HPLC. The amount of CPFX bound to the polymer was calculated by
subtracting free concentration from the initial CPFX concentration. The results were used
for the selectivity calculation.
Static distribution
coefficient KD and separation factor a
were
KD=CP/CS (1)
CP is the concentration of substrates, on the polymer (mmol/g); CS is the concentration of substrates, in solution (mmol/mL). a represents the proportion of the distribution coefficients (KD) of the MIP and the NIP.
a= KDMIP/ KDNIP (2)
Table.2 Selectivity adsorption of MIP for substrates
SMIPs |
Analyte |
CS ( mmol/mL) |
CP( mmol/g) |
KD(mL/g) |
a |
MIP |
CPFX |
166.98 |
135.14 |
0.81 |
2.03 |
NIP |
CPFX |
262.40 |
39.72 |
0.15 |
0.63 |
Table2 shows that MIP exhibits a good recognition selectivity for CPFX, and the separation factor of MIP (a=2.03) is much higher than that of NIP (a=0.63). The obvious difference of binding selectivity between MIP and NIP may result from locations of the carboxyl functional groups within the micro-cavities created in the MIP and NIP matrix by imprinting, and can be explained by imprinting process. In the MIP, the position functional groups are regular and corresponding to structure of imprinted template, but irregular in the NIP.
3.4. Adsorption isothermal line of MIP
Fig.2 shows that the change of QMIP value tend to flat with the
concentration of CPFX increasing, which indicates that the binding sites of the MIP are
gradually saturated by CPFX molecule. However, QNIP value of NIP is lower than
QMIP, and do not increase with the concentration of CPFX after 0.5mmol/L. This
indicates the binding sites are small in number and lack in selectivity inside of NIP.
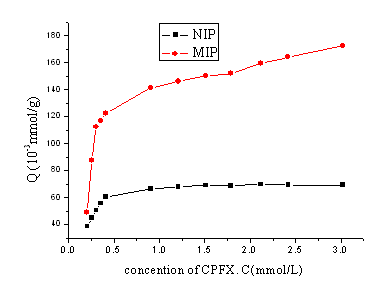
Fig.2 The binding isotherm of MIP and NIP for CPFX at 25oC
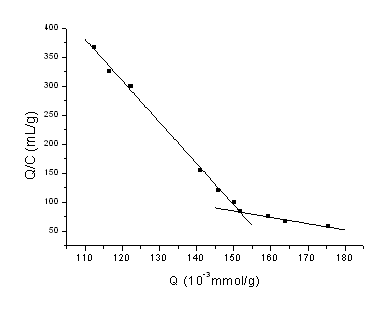
Fig.3 Scatachard plots of MIP for CPFX
The binding isotherm of MIP
was analyzed by Scatchard equation [10] as follows:
Q/C = (Qmax -Q)/Kd. (3)
In this equation: Q is the binding capacity; C is the concentration of
CPFX (mmol/L); Qmax is the maximum apparent binding capacity; Kd
is the dissociation constant at binding site. The dissociation constants at binding sites
are 1.35x10-4mol/L and 1.19x10-3 mol/L respectively.
The Scatachard plot of Q/C vs Q was shown in Fig.3. As seen from Fig.3,
the relationship between Q/C and Q is non-linear, that is to say the binding sites in MIP
are heterogeneous in respect to the affinity for CPFX. The two sections of plot can be
regarded as two pieces of straight lines, which indicates that two kinds of binding sites
in MIP are mainly created. In addition, we can infer that two kinds of heterogeneous
binding sites inside of MIP locating at the point that carboxyl and -NH on the piperidic
ring of CPFX molecule interact with carboxyl of MAA through non-covalent.
4. CONCLUSION
Ciprofloxacin-imprinted polymers (MIPs) were prepared by bulk polymerization with MAA
as functional monomer and TRIM as cross linker. The MIPs exhibit high binding capacity and
good recognition selectivity for CPFX, and separation factor a
is 2.03. The mechanisms of molecular imprinting and molecular
recognition of CPFX are described that two kinds of specific recognition sites for acidic
CPFX can be created within the MIP.
REFERENCES
[1] Lars I. Andersson, Journal of Chromatography B, 2000, 745: 3.
[2] Li Z W, Liu S B, Yang G L, Li B Z, Chen Y. Chinese Journal of Chromatography,
2005, 23: 622.
[3] Greene N T, Shimizu K. J. Am.Chem.Soc, 2005, 127: 5695.
[4] Lele B S, Kulkarni M G, Mashelkar R A. React Funct
Polym, 1999, 39: 37.
[5] Hernández M, Borrull F, Calull M, Trends Anal.Chem, 2003, 22: 416.
[6] Sultan S M, Suliman F E O. Analyst, 1992, 117: 1523.
[7] Du X Y, Peng T, Li J S. Chinese J. Anal.Chem, 2003, 31: 720.
[8] Guo H.S., He X.W., Zhou J, et al. Chin.J.Anal.Chem, 2001, 29(2): 128.
[9] Caro E, Marcé R M, Cormack P A G, et al. Anal.Chim.Acta, 2006, 562: 145.
[10] Matsui J., Miyoshi Y., Doblhoff-Dier O., et al. Anal.Chem, 1995, 67: 4404.
MAA/TRIM
分子印迹聚合物的合成和对环丙沙星的吸附选择性
高俊刚,刘占理,刘芃岩 姜宁
(河北大学化学与环境科学学院,保定市,071002)
摘要
本文以环丙沙星为印迹分子,甲基丙烯酸为功能单体,甲基丙烯酸三羟甲基丙烷酯为交联剂合成了分子印迹聚合物,用扫描电子显微镜进行了表征。用等温吸附法研究了该分子印迹聚合物的吸附容量及对沙星类药物的吸附选择性和吸附机理。结果表明该分子印迹聚合物对模板化合物有很好的分离选择性,分离系数为2.03。
关键词 分子印迹聚合物,环丙沙星,分子识别