http://www.chemistrymag.org/cji/2007/092005ne.htm |
Feb. 2,
2007 Vol.9 No.2 P.5 Copyright |
On-line solid phase microextraction of jatrorrhizine in plasma with poly (methacrylic acid–ethylene glycol dimethacrylate) monolith
Wang Manman, Yang Gengliang , Liu Haiyan
(College of Pharmacy, Hebei University, Baoding 071002, Institute of Chemistry, Chinese
Academy of Sciences, Beijing 100080, China)
Received Nov. 16, 2006.
Abstract The poly (methacrylic acid–ethylene glycol dimethacrylate) monolith was developed for on-line
determination of trace-level jatrorrhizine in plasma. Jatrorrhizine in human plasma was
determined without any other manipulation except centrifugation. The average recovery of
jatrorrhizine in plasma analysis was 87.2 %. Intra-day and inter-day precisions for
jatrorrhizine were 1.6 % and 6.2 % respectively. The method was linear over the range of
3.60 to 360 ng/mL and the detection limit was 0.86 ng/mL. The proposed method was
demonstrated to cope robustly with the extraction and analysis of jatrorrhizine in plasma
samples by direct injection.
Keyword Solid phase microextraction; Enrichment; Plasma; Jatrorrhizine; Monolith
1 INTRODUCTION
In clinical and pharmaceutical analysis,
the screening and determination of drugs in body fluids play an important role in
pharmacokinetics [1]. Therefore the sample clean-up and enrichment steps are
frequently necessary. Solid phase microextraction (SPME) is developed as an attractive
preconcentration technique since it has gained the widest acceptance due to the ease of
automation, the high analyte recovery and the avoidance of solvent while providing
accurate and reproducible results [2-7]
The poly (methacrylic acid-ethylene glycol dimethacrylate) monolithic capillary column was first introduced for in-tube SPME by Feng and coworkers and successfully applied to the determination of drugs in serum and urine samples [8-11].
Jatrorrhizine is one of the bioactive components isolated from a number of medicinal plants, such as Tinospora cordifolia (roots), Berberis julianae Schneid and Jatrorrhiza palmate Miers. It displays a great variety of biological and pharmacological activities (e.g. antibacterial, antifungal and parasite-fighting properties) [12]. So the development of a sensitive method to determine jatrorrhizine in biological fluids was important in toxicological, forensic and pharmaceutical research. Zou Hanfa et al [13] had described the method of capillary electrochromatography to determine jatrorrhizine in plasma by liquid-liquid extraction.
In this study, the monolith was applied in SPME-HPLC system for the enrichment and determination of jatrorrhizine in human plasma.
2. EXPERIMENTAL
2.1. Chemicals and materials
a-Methacrylic
acid (MAA) was purchased from the First Tianjin Chemical Reagent Factory (Tianjin, China)
and ethylene dimethacrylate (EDMA) was from Acros (New Jersey, USA). 2,
2'-Azobisisobutyronitrile (AIBN) was obtained from Beijing Chemical Reagent Company
(China). Jatrorrhizine was obtained from National Institute for the Control of
Pharmaceutical and Biological Products. Double distilled water was used for the
experiment. All of the solutions and samples were filtered through a 0.45 mm membrane filter
(Millipore) before use.
Jatrorrhizine was prepared as 0.18 mg/mL solution in methanol. Plasma samples were collected from drug-free, healthy
volunteer. Any precipitated material was removed by centrifuging the sample at 5000 rpm
for 10 min. The supernatant of the plasma was directly spiked with
jatrorrhizine solution and then diluted five times with 0.05mol/L phosphate buffer (pH
4.5) as working solution.
2.2. Instrument and SPME-HPLC procedure
The HPLC system consisted of Agilent 1100 Series components (Agilent Technologies,
Palo Alto,USA) including a quaternary pump, an on-line degasser, an auto-sampler, a
variable-wavelength UV detector and an Agilent 1100 chemstation. The chromatographic
analysis operated at 265nm was achieved using a C18 column (DiamonsilTM,
5 mm, 250×4.6
mm, Dikma Technologies) at the flow rate of 1.0 mL/min. The optimized mobile phase was
0.05 mol/L phosphate buffer (pH 3.0)-acetonitrile (50:50, V: V) containing
1.60 g sodium dodecyl sulfate per 100mL. SPME column was placed in the sample-loop
position of the value and used for sample pretreatment. In the "load" position, 1.0 mL of each working
solution was injected into the SPME column. 0.05 mol/L phosphate buffer (PB) (pH 4.5) was
passed through the SPME column with the valve switched to the "injection" position to deproteinize. After
that, the "load" position was set and the analysis mobile phase was employed through the
analytical column to obtain a stable baseline for chromatographic separation. Then,
desorption and separation processes were achieved by simply switching the valve to the
"injection" position.
2.3. Preparation of poly (MAA–EDMA) monolithic support
The monolithic column was prepared by an "in-situ" polymerization within the confines of a stainless-steel
microtube column (65×1.5 mm I.D.) [8].
3. RESULTS AND DISSCUSSION
3.1 The extract of jatrorrhizine on the poly (MAA-EDMA) monolith
Since poly (MAA–EDMA)
monolithic material possesses polar groups and carboxyl groups in the hydrophobic bone
structure, the results displayed jatrorrhizine could be extracted on the poly (MAA–EDMA) monolith. Jatrorrhizine attributes to the quaternary alkaloid
with the pKa values below 9.0 [13]. An adsorption process was thought to
be involved in the extraction procedure. The ion exchange (acid–base) interaction and the hydrophobic interaction were regarded as
the two main factors that dominated the retention of the analytes on the monolithic
column.
3.2 The effect of flow rate
In addition, the flow rate of SPME procedure was
investigated from 0.05 to 2.0 mL/min. The results showed that it was optimized below 0.5
mL/min, and just a slight increase of the extraction efficiency was found when reducing
the flow rate. Therefore, 0.5 mL/min was selected to offer both shorter extraction time
and satisfying extraction efficiency.
3.3 The analysis of plasma sample
Plasma samples were analyzed using the previously
determined conditions. Fig.1 displayed the chromatograms obtained for SPME-HPLC analysis.
During the whole process of plasma analysis, the increase of backpressure of the
monolithic and the analytical column had not been observed, indicating that no matrix
irreversible adsorption occurred.
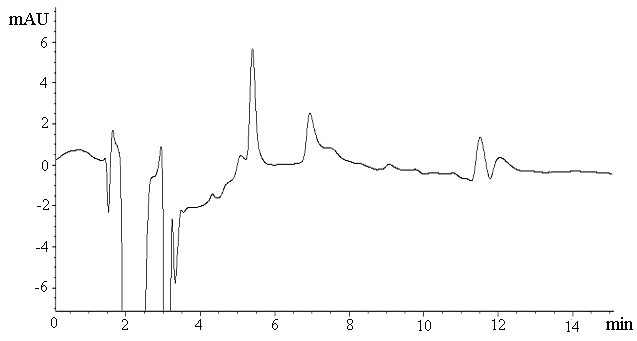
(a)
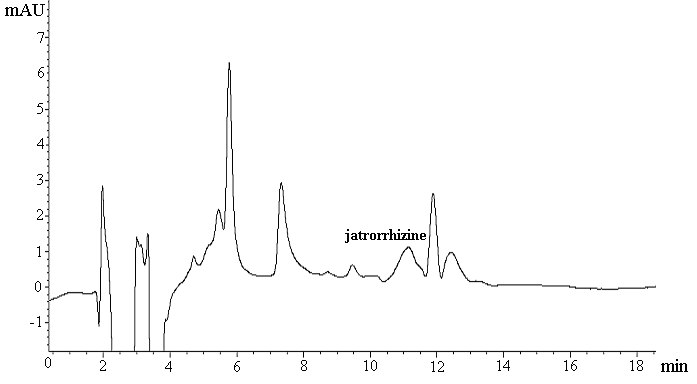
(b)
Fig. 1 Chromatograms obtained by SPME of
blank plasma sample (a) and spiked plasma sample at 4.5ng/mL with 1.0 mL injection volume
(b)
The extraction recoveries of jatrorrhizine were shown in Table1 and the average of recovery was 87.2 %. Successful extraction was accomplished with the satisfying regression coefficient (0.97) for the calibration curves obtained over a range of 3.6 to 360 ng/mL. The limit of detection (LOD) and limit of quantification (LOQ) were determined as 0.86 and 2.9 ng/mL at a concentration where the signal to noise ratio was equal to 3 and 10, respectively. Intra-day (n=5) and inter-day (n=3) precisions for jatrorrhizine were 1.6% and 6.2% respectively. Moreover, the monolith is very stable, since no significant changes were observed in results from extractions performed over two months during this study.
Table 1 The recovery of jatrorrhizine in plasma sampleAdded amount ( mg) |
Detection amount ( mg) |
Recovery (%) |
0.6500 |
0.5642 |
86.8 |
0.9750 |
0.8404 |
86.2 |
0.008 |
0.0709 |
88.6 |
4. CONCLUSIONS
The poly (methacrylic acid–ethylene glycol dimethacrylate)
medium was applied for on-line determination of jatrorrhizine in plasma sample. Compared
with the methods previously reported, plasma samples could be extracted without any other
manipulation except centrifugation; meanwhile trace-level jatrorrhizine could be enriched
effectively. And the SPME process required no organic solvent.
ACKNOWLEDGEMENT This work was supported by the National Natural Science Foundation of China (No.20375010), "BaiRen Project" of Chinese Academy of Science.
REFERENCES
[1] Wang W X, Wang A G, Wang J C,
Clinical Pharmacokinetics. Beijing: Xueyuan Press, 1998
[2] Fritz J S, Mack M, J. Chromatogr.
A, 2000, 902, 137.
[3] Pawliszyn J, Solid Phase Microextraction-Theory and Practice, New York: Wiley- VCH,
1997.
[4] Smith R M, J. Chromatogr. A, 2003, 1000, 3.
[5] Raynie D E, Anal. Chem. 2006, 78, 3997.
[6] Svec F, Huber C G, Anal. Chem. 2006, 78, 2101.
[7] Svec F, J. Chromatogr.B, 2006, 841, 52.
[8] Fan Y, Feng Y Q, Da S L et al, Anal. Chim. Acta, 2004, 523, 251.
[9] Fan Y, Feng Y Q, Da S Let al, Analyst, 2004, 129 , 1065.
[10] Wen Y, Fan Y, Zhang M et al, Anal. Bioanal. Chem. 2005, 382, 204.
[11] Fan Y, Feng Y Q, Zhang J T et al, J. Chromatogr.A, 2005, 1074 , 9.
[12] Xiao P G, Modern Chinese Materia Medica, Beijing : Chemical Industry Press, 2002.
[13] Dong J, Xie C H, Tian R J et al, Electrophoresis, 2005, 26, 3452.
聚甲基丙烯酸-乙二醇二甲基丙烯酸酯整体柱在线固相微萃取血浆中的药根碱
王曼曼 杨更亮 刘海燕
(河北大学药学院 保定 071002 中国科学院化学所 北京 100080)
摘要 利用聚甲基丙烯酸-乙二醇二甲基丙烯酸酯整体柱在线固相微萃取血浆中低含量药物药根碱。该法不需要引入复杂的样品制备操作。数据显示:方法回收率为87.2%,日内和日间精密度分别为1.6%和6.2%。其检出限0.86
ng/mL,且方法在3.60-360 ng/mL线性范围良好。实验证明本文的方法可以利用直接进样来处理血浆中的药根碱。
关键词 固相微萃取 富集 血浆 药根碱 整体柱