http://www.chemistrymag.org/cji/2007/094016pe.htm |
Apr. 2,
2007 Vol.9 No.4 P.16 Copyright |
Huo
Guoyan, Song Dayong, Gao Yangai
(College of Chemistry and Environmental Science, Hebei University, Hebei Baoding, 071002 )
Abstract The perovskite
compound BiFeO3 was synthesized by sol-gel method. The compound was
investigated by XRD and the results show that its crystal structure belongs to
rhombohedral system with space group R3c. This compound was used as photocatalyst for the
photocatalytic degradation of methyl orange. The influence of the acidity of solution, the
amounts of photocatalyst, the concentration of methyl orange and irradiation time of
sunlight on the efficiency of degradation have been studied. A mechanism related to the
process of photocalysis is proposed. The results show that BiFeO3 possesses
photocatalytic activity.
Keywords Perovskite compound BiFeO3 Methyl orange Photocatalysis Degradation
1. INTRODUCTION
In recent years, many attentions have
been focused on the semiconductor-based photocatalytic materials for degradating toxicants
as they can be induced electron-hole pairs on their surface or inner of them by solar
radiation[1-2]. The generated electrons and holes possess strong reduction and
oxidation abilities, respectively, which both can react with H2O molecules and
OH- ions adsorbed on the surface of semiconductor-based photocatalytic
materials particles and finally generate hydroxyl radicals[3]. In many
photocatalytic materials, TiO2 powder is considered to be a very efficient
catalyst that is non-toxic and stable. However, due to its wide bandgap (3.2eV), it is
only suitable for work using ultraviolet light as the energy source[4], such as
high pressure mercury lamp and less than 5% of the sunlight on the earth. Recently,
perovskite-type compounds are employed for the degradation organic pollutants in the
wastewater owing to their narrower bandgap which can be easily excited under visible light
irradiation[5].
Bismuth-containing
oxides are of considerable importance as heterogeneous catalysts for a variety of
processes including the oxidative coupling of methane, the ammoxidation of propylence, and
the oxidation of hydrocarbons, carbohydrates and CO[6-9]. Based on the
advantage of bismuth-containing oxides and perovskite-type compounds, the photocatalytic
behavours which BiFeO3 decolours methyl orange have been studied.
2 EXPERIMENTAL
2.1 Apparatus and reagents
Y-2000 X-ray diffractometer (Aolong X-ray Apparatus Ltd. Corporation, Dandong),
UV-Vis8500 spectrometer (Tianmei Scientific Apparatus Ltd. Corporation, Shanghai), pH-3C
acidimeter (Leici Analytic Apparatus Factory, Shanghai), DHG-9023A oven (Shanghai Exact
Apparatus and Meter Ltd. Coporation, Shanghai)
Methyl orange (analytical reagent grade), Sodium hydroxy (analytical
reagent grade), hydric sulphate (analytical reagent grade)
2.2 Synthesis of BiFeO3
An amount of Bi(NO3)3 (99.9%) and Fe(NO3)3
(99.5%) was dissolved in a diluted HNO3 solution with stirring and following
then citric acid was added to the mixture. Under stirring condition the above mixture
solution was heated until it formed dry gel. BiFeO3 was prepared by hand-mixing
dry gel in mortar. Then they were pressed into pellets under 10Mpa pressure for 1 min
following then preheating at 500°C for 6h. The sample was annealed in air at 750°C for 16h and it was cooled to room
temperature in an oven. Phase analysis and characterization were carried out by Y-2000
X-ray diffractometer.
2.3 Experimental processes and data collection
The maximum absorbance of the methyl orange was obtained by using UV-Vis8500 spectrometer
from 180 to 1000nm (see Table 1). The maximum absorbance of methyl orange solution is
obtained at 507nm when the pH value of initial methyl orange solution is less than 3.1 and
the maximum absorbance of initial orange solution is measured at 464nm when the pH value
of methyl methyl orange solution is larger than 4.4. An amount of BiFeO3 was
added to a certain concentration methyl orange solution at a given pH value and following
then it was exposed to solar light for several hours. Then, the photocatalyst was
filtered. The degradation ratio was calculated based on formula: D = [(A0 –
At)/A0]*100%, where A0 is
the absorbance of original methyl orange solution and At is the absorbance of
methyl orange solution measured after finishing photocatalyzed reaction.
Table 1 Maximum absorbable wavelength of methyl orange under different pH
pH value of methyl orange solution |
wavelength of maximun absorbance |
pH ≥ 4.4 pH ≤ 3.1 |
192.0nm; 464.0nm 189.0nm; 507nm |
3 RESULTS AND DISCUSSION
3.1 Crystal structure of BiFeO3
The sample cooled from the sintering temperature was characterized by X-ray powder
diffraction using Cu Kα radiation at room
temperature. Fig. 1 shows the XRD pattern of BiFeO3 and the compound BiFeO3
can be attributed to rhombohedra system with lattice parameters a = 5.5752(8) and c =
13.8552(25) in a hexagonal cell setting and the space group R3c agreed with the literature
reported[10]. The observed reflections of BiFeO3 are in accord with
those calculated using the CaRIne program.
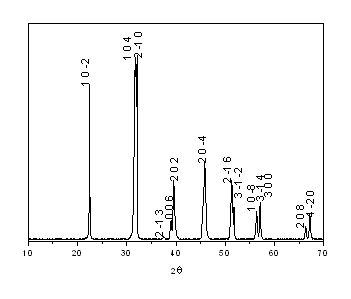
Fig. 1 X-ray diffraction pattern of BiFeO3 compound
3.2 Effect of solution acidity on
degradation ratio of methyl orange
Different pH values of methyl orange solution, 1mg/ dm3, 100cm3,
was obtained by adding a 2.0mol/dm3 NaOH solution or by adding a 2.0mol/ dm3
H2SO4 solution and the absorbance of each solution was measured.
Then, photocatalyst, BiFeO3, 50mg, was introduced into the solutions mentioned
above. Following they were exposed to sunlight for 4 hours from 11:00 to 15:00 in summer.
Subsequently, these solutions were filtered and the absorbances were measured by using
UV-Vis8500 spectrometer. Table 2 represents the influence of original solution acidity on
the degradation ratio of methyl orange. It can be seen from Table 2 that optimum acidity
of original methyl orange is around 2.0 in pH value and in this pH value the decgradation
ratio is up to 99.3%. This can be assigned to different structure of methyl orange in
different acidity. The methyl orange structure transition with acidity of solution can be
showed as the following:
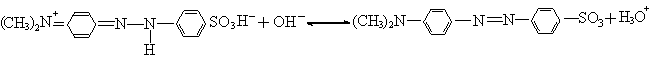
pH ≤ 3.1, quinonoid structure pH ≥ 4.4, azo-type structure
Table 2 Effect of acidity of solution on degradation ratio of methyl orange
Acidity (pH) |
2.17 |
3.03 | 6.97 | 9.35 | 11.75 |
Degradation ratio (%) |
99.3 |
67.8 | 47.5 | 21.8 | 13.8 |
It indicates that quinonoid methyl orange can easily be degradated by BiFeO3 photocatalysis.
3.3 Effect of methyl orange
concentration on the degradation ratio of methyl orange
Photocatalyst, BiFeO3, 4×100mg, was added to various concentrations of 100cm3
methyl orange solution of pH= 2 adjusted by 2mol/dm3 H2SO4,
1mg/dm3, 5mg/dm3, 10mg/dm3 and 15mg/dm3,
respectively and following then they were exposed to solar light 4 for hours from 11:00 to
15:00 in summer. Denpendence of degradation of methyl orange on the concentration of
methyl orange is shown in Fig. 2. It can be seen from Fig. 2 that degradation ratio of
methyl orange decrease with the concetration of methyl orange. When the concentration of
methyl orange is less than 5mg/dm3 the degradation ratio decreases slowly and
is more than 85%. However, when the concentration of methyl orange is higher than 5mg/dm3
the degradation ratio decreases rapidly. This can be assigned to that the degradation
products of methyl orange are absorbed on the surfaces of photocatalyst and the absorbate
runs off difficultly.
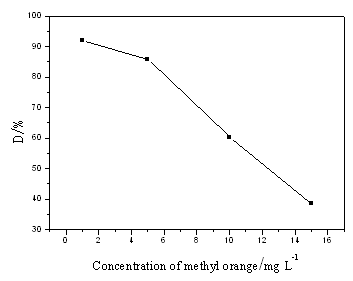
Fig.2 Denpendence of degradation of methyl
orange on original concentration of methyl orange
3.4 Effect of amount of photocatalyst
on the degradation ratio of methyl orange
Different amounts of BiFeO3, 40mg, 60mg, 80mg and 100mg, were added to 100cm3,
5mg/dm3, methyl orange solution of pH » 2 adjusted by 2mol/dm3
H2SO4, respectively. Afterwards, they were exposed to sunlight for 4
hours from 11:00 to 15:00 in summer. Dependence of degradation of methyl orange on the
amount of photocatalyst, BiFeO3, is shown in Fig. 3. It can be seen from Fig. 3
that degradation ratio of methyl orange increases rapidly and that degradation ratio is up
to 93% when the amount of photocatalyst is more than 80mg in 200cm3 methyl
orange solution.
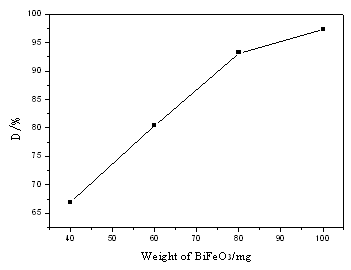
Fig.3 Effect of amount of BiFeO3
on degradation of methyl orange
3.5 Effect of irradiation time in
sunlight on the degradation ratio of methyl orange
100mg photocatalyst, BiFeO3, was added to 100cm3, 5mg/dm3,
methyl orange solution of pH » 2 adjusted by 2mol/dm3 H2SO4.
Subsequently, the solution was irradiated for different times in solar light. The
dependence of degradation ratio of methyl orange on the irradiation time is presented in
Fig. 4. It can be seen from Fig. 4 that the degradation ratio of methyl orange increases
with irradiation time. From experimental result it can be derived that the degradation
products are absorbed on the surfaces of photocatalyst, BiFeO3, and the
absorbate escapes slowly from photocatalyst. Therefore, escaping the products of
degradation methyl orange from the surface of photocatalyst, BiFeO3, is the
determining-step in this reaction.
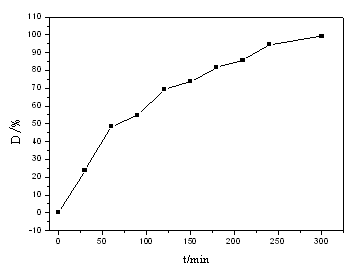
Fig. 4 Dependence of degradation ratio of methyl orange on the
irradiation time
3.6 Reaction mechanism
The acidity-dependent degradation can mainly be attributed to the variations of surface
charge properties of the photocatalyst. Afterward, this condition changes the absorbing
behaviour of methyl orange on the photocatalyst surface. Since methyl orange has an
anionic configuration its adsorption is preferred in an acidic solution. One hand, this
increases methyl orange in close contact with the catalyst and enhances its oxidative
degradation by positive holes or hydroxyl radicals upon photoexcitation. On the other
hand, quinonoid structure of methyl orange is unstable. Based on the experimental results
and analysis mentioned above, a reaction mechanism was proposed as follows:
Photo-catalyst ![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)
where MO and MM represent methyl orange and micromolecular substance, respectively.
ACKNOWLEDGEMENTS This work was supported by Foundation for Doctorate, Education Department of Hebei Province (B2002102), China.
REFERENCES
[1] Yu Zhiyong, Mielczarski E, Mielczarski J A, et al., Stabilization mechanism of TiO2
on flexible fluorocarbon films as a functional photocatalyst. J. Mol. Catal. A: Chem.
2006, 260: 227–234.
[2] Kusvuran E, Samil A, Atanur O M, et al. Photocatalytic degradation of di- and
tri-substituted phenolic compounds in aqueous solution by TiO2/UV. Appl. Catal.
B: Environ. 2005, 58: 211–216.
[3] Sakkas V A, Lambropoulou D A, Sakellarides T M, et al. Application of solid-phase
microextraction for monitoring the photocatalytic decomposition of fenthion and parathion
in aqueous TiO2 suspension. Anal. Chim. Acta 2002, 467: 233–243.
[4] Jiang Y, Zhang P, Liu Z, et al. The preparation of porous nano-TiO2 with
high activity and the discussion of the cooperation photocatalysis mechanism. Mater. Chem.
Phys. 2006, 99: 498–504.
[5] Yang Y, Sun Y, Jiang Y, Structure and photocatalytic property of perovskite and
perovskite-related compounds. Mater. Chem. Phys.
2006, 96: 234–239.
[6] Mallat T, Bodnar Z, Hug P, et al. Selective Oxidation of Cinnamyl Alcohol to
Cinnamaldehyde with Air over Bi-Pt/Alumina Catalysts. J. Catal. 1995, 153: 131–143.
[7] Besson M, Lahmer F, Gallezot P, et al. Catalytic Oxidation of Glucose on
Bismuth-Promoted Palladium Catalysts. J. Catal. 1995, 152: 116–121.
[8] Felthouse T R, Fraundorf P B, Friedman R M, et al. Expanded lattice ruthenium
pyrochlore oxide catalysts I. Liquid-phase oxidations of vicinal diols, primary alcohols,
and related substrates with molecular oxygen. J.
Catal. 1991, 127: 393–420.
[9] Hucknall D J, Selective Oxidation of Hydrocarbons, Academic Press, New York, 1974.
[10] Sosnowska I, Przenioslo R, Fischer P, Murashov, Neutron diffraction studies of the
crystal and magnetic structures of BiFeO3 and Bi0.93La0.07FeO3.
J. Magn. Magn. Mater. 1996, 160: 384–385.
霍国燕 宋大勇 高艳改
(河北大学化学与环境科学学院,河北 保定,071002)
摘要 用溶胶凝胶法合成钙钛矿型BiFeO3化合物,并用X射线衍射仪(XRD)对产物进行物相分析:样品属于菱方晶系,空间群为R3c. 以此化合物为光催化剂研究了溶液酸度、光催化剂的量、光照时间对甲基橙降解率的影响以及甲基橙浓度对其光催化降解率的影响,结果表明:BiFeO3光催化降解甲基橙溶液的最佳酸度是pH值在2.0左右;催化剂BiFeO3的最佳质量浓度为1.00 g/L,并且随着甲基橙浓度的增大其降解率降低;随着光照时间的增加,甲基橙的脱色率增加,并提出了光解的可能机理。
关键词 钙钛矿型化合物BiFeO3 甲基橙 光催化 降解率