http://www.chemistrymag.org/cji/2007/094018ne.htm |
Apr. 2,
2007 Vol.9 No.4 P.18 Copyright |
Synthesis of new Schiff bases of vanillin derivatives
Zhu Wanren, Huang Zhiwei, Li Jiagui, Chen Yuan, Yan Quan, Hu Peizhi#, Wu Chengtai#(Department of Chemistry and Biology ,Yulin Teache's College, Yulin 537000, China ; #College of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072 China )
Received Dec. 26, 2006.
Abstract Two intermediates and four
new Schiff bases were synthesized. The two intermediates were prepared from vanillin and
1-butylbromide or 2-chloroacetic acid, respectively. Condensation of 3-methoxy-
4-butoxybenzaldehyde with 2-amino-4-methylphenol or 2-aminophenylmethanol and
3-methoxy-4-carboxy- methylbenzaldehyde with aminoacetic acid or 2-amino-4-methylphenol
gave the four Schiff bases. All of the compounds were characterized by element analysis,
UV, IR, 1H NMR and 13C NMR.
Keyword vanillin, Schiff bases, synthesis
1. INTRODUCTION
Schiff bases are compounds of containing C=N group. They are often synthesized from amine and aldehyde or ketone. To synthesis of new Schiff bases and their complexes and investigation of their properties have been being a popular theme[14~18]due to their strong coordination capability and diverse biological activities, such as antibacterial, antitumor activities etc. [1~6,9~13,16]. However, few were reported on new Schiff bases of vanillin derivatives. In view of above, it is reported here in the synthesis of new Schiff bases containing vanillin derivatives. The new four Shiff bases were synthesized by condensation reaction of the intermediates, 3-methoxy-4-butoxy- benzaldehyde and 3-methoxy-4-carboxymethylbenzaldehyde with three kinds of amines respectively. The general synthesis route of the new Schiff bases is shown in Scheme 1. They have been characterized by elemental analysis, IR, UV-VIS, 1H NMR and 1C NMR.
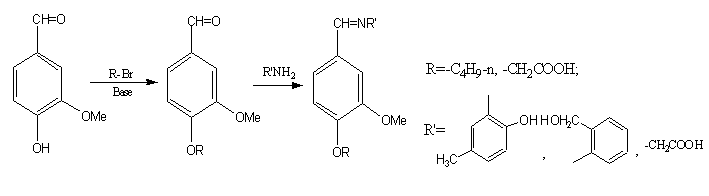
Scheme 1 The general synthesis route of the new Schiff bases 2 EXPERIMENTAL
2.1 Instruments and materials
Reagents: vanillin(A.R), NaOH(A.R), anhydrous ethanol(A.R), o-amino-p-methylphenol (A.R),o-aminobenzyl alcohol(A.R), 1-brorobutane(A.R), glycine(A.R.).
The IR spectra in the 4000-400cm-1 in KBr pellets were obtained on a Nicole FTIR instrument. Elemental analysis was determined by PE2400II Elemental Autoanalyzer. UV- VIS spectra were recorded on a Tu-1901 Spectrophotometer. 1H NMR and 13C NMR spectra were acquired at a AVANCE AV 500MHz Spectrometer for CDCl3 solution, using TMS as internal standard. Melting point was determined by WRS-1 Digital Melting-Pointer.
2.2 Synthesis of 3-methoxy-4-butoxybenzaldehyde(I)
Vanillin(15mmol) was stirred with 1-bromobutane(30mmol) and sodium hydroxide(15mmol) in ethanol at 50~60°C, tetrabutylammonium iodide as catalyst, for 5h under nitrogen atmosphere. After that, the majority of solvent was removed under reduced pressure. The crude product was extracted with benzene, washed by 5%~10% sodium carbonate solution, dried with anhydrous magnesium sulfate. Then it was distilled under reduced pressure and the light yellow liquid fraction of 205°C-210°C/0.095Mpa was collected. Yield: 67.3%,
2.3 Synthesis of 3-methoxy-4-carboxymethylbenzaldehyde(II)
In a 50ml conical flask, Vanillin 3g(0.02mol), ethanol 4ml, and potassium hydroxide solution 5ml(1.1g,0.02mol) were stirred. Potassium chloroacetate solution(0.022mol) was added dropwise. The pH of mixture liquid was adjusted with triethylamine. The mixture was refluxed for 120min under nitrogen atmosphere. This reaction mixture was allowed to cool to room temperature, then poured onto crushed ice, and added concentrated hydrogen chloride to pH=1. The solid was filtered under reduced pressure, washed by ice water. The crude product was recrystallized from 40% ethanol solution to give the yellowish brown color crystal(2.7g, yield 64.3%). m.p. 177.2~177.9°C. 1H NMR(CDCl3)d: 13.56 (s,1H,-COOH), 9.85(s,1H,-CH=O), 7.06~7.53(m,3H, 7.7Hz,Ar-H), 4.84(s,2H,-CH2-), 3.86(s,3H, -CH3)ppm; 13C NMR(CDCl3): 191.8(-CH=O), 170.0(-COOH), 153.0, 149.7,130.6,126.0, 112.9, 110.7(-Ar), 65.4(-CH2-), 56.1(-CH3) ppm; IR(KBr)n: 2958(w,-COOH), 1715(C=O), 1645, 1581,1509,1467,1424(Ar,C=C),1375(-CH3),1282,1268,1223,1134(C-O-C)cm-1; UV(λmax/nm)(Abs):306.0(0.9374), 274.0(1.1995), 228.0(1.7254), 206.0(1.6256)。Anal. for C10H10O5(210.18) calcd:C,56.84(57.15); H,4.71(4.80).
2.4 Synthesis of Schiff bases (III)
In a 50ml conical flask, 5mmol of intermediate(I) , 2-amino-4-methylphenol 5mmol and anhydrous ethanol 20ml was adjusted pH from 7 to 8 by triethylamine and was reacted at 50°C~60°C for 13h under nitrogen atmosphere. After a part of solvent was removed, the residue was kept in refrigerator over a day. The precipitate was filtered out. Recrystallization from anhydrous ethanol afforded Schiff base(I) 0.73 g(46.6% yield) as yellow crystal, mp 86.4~87.0°C.1H NMR(CDCl3)d: 13.10(s,1H,Ar-OH), 8.56(s,1H.-CH=O), 6.92~7.57(m,6H,Ar-H), 4.10~4.13(t,2H,-OCH2-), 3.96(s, 3H,-OCH3), 2.34(s,3H,Ar-CH3), 1.87~2.34(m,2H,-CH2-), 1.53~1.57(m,2H,-CH2-), 1.01~1.04(t,3H,-CH3)ppm; 13C NMR (CDCl3): 156.9(-CH=N), 152.1,149.9,149.8, 135.8,129.3,129.1,128.8,124.3, 116.5,114.5,112.0,109.7(Ar-), 68.8 (-OCH2-),56.1(-OCH3), 31.1(Ar-CH3), 20.8, 19.2,13.8(-CH2CH2 -CH3)ppm。IR(KBr)n:3395(w,O-H), 1626(C=N), 1615,1599,1580(Ar,C=C),1375 (-CH3),1271,1258,1236,1163,1144,1066,1029(C-O-C)cm-1; UV(lmax/nm)(Abs):354.0(0.9090), 284.0(0.8300), 208.0(1.7492). Anal. for C19H23NO3 (313.19) calcd :C,72.77(72.82); H,7.21(7.40); N,4.28(4.47).
2.5 Synthesis of Schiff base(IV)
The appropriate intermediate (I) of 5mmol in 20ml ethanol were added 2-aminophenylmethanol(5mmol). Then, the pH was adjusted to 7~8 by triethylamine. The reaction mixture was stirred at 50°C~60°C for 32h under nitrogen. Then, a majority of solvent was removed under reduced pressure. After three days, white crystals were filtrated off and recrystallized from ethanol to give white prisms(0.31g, 19.80%),m.p. 85.6~86.1°C. 1H NMR(CDCl3)d: 8.42(s,1H,-CH=O),7.54~6.72 (m,7H,Ar-H), 4.14(s,2H,-CH2-O), 4.15 ~4.13 (t,2H,-OCH2-R) , 3.98(s,1H,-OH), 3.92(s,3H,-OCH3), 1.89~1.84(m,2H,-CH2-R), 1.55~1.50(m,2H, -CH2-CH3), 1.04~0.99(t,3H,-CH3) ppm; 13C NMR (CDCl3): 159.4(-CH=N), 149.8,141.8,131.9,127.5,124.4,122.0, 119.7, 119.2,117.6,116.8,112.8, 109.8(Ar), 68.9(-OCH2-R),64.2(-CH2OH), 56.1(-OCH3), 20.8 (-CH2- R), 19.2(-CH2-), 13.8(-CH3) ppm。IR(KBr)n:3329(w,O-H), 1608(C=N), 1592,1519(Ar,C=C), 1374(-CH3), 1265,1228,1163, 1138,1088, 1050,1027 (C-O-C)cm-1; UV(lmax/nm)(Abs):278.0(0.3154), 236.0(0.8300), 208.0(3.1003)。Anal. for C19H23NO3 (313.19) calcd :C73.04(72.82); H,7.89(7.40); N,4.24(4.47)。
2.6 Synthesis of Schiff base(V)
3-methoxy-4-carboxylmethoxybenzaldehyde(1g, 5mmol) was added with aminoacetic acid in 60% ethanol and the pH was adjusted to 7~8. The reaction mixture was stirred at 50℃~60℃ for 48h under nitrogen. The solvent was removed 50% under reduce pressure. Yellow solid was precipitated. The resulting solid was filtrated off and washed with anhydrous ethanol and anhydrous ether successively to give yellow powder(0.54g, 40.3%), m.p.171.0~172.6°C.1H NMR(CDCl3)d:13.82(s,2H,-COOH), 9.90 (s,1H,-CH=N), 7.55~7.12(m,3H,Ar-H), 4.90(s,2H,-OCH2-), 3.94(s,2H,=N-CH2-), 2.07(s,3H, -OCH3)ppm; 13C NMR(CDCl3): 190.8,190.0(-COOH), 168.7(-CH=N), 153.0,150.1,131.2,125.2, 112.9,110.4(-Ar), 68.3(=N-CH2-), 65.0(-OCH2-),55.4(-OCH3)ppm。IR(KBr)n:3485(w,COOH), 1648,1645(C=N), 1581,1509(Ar,C=C), 1375(-CH3), 1282,1267,1221,1134 (C-O-C)cm-1; UV(lmax/nm)(Abs):310.0(0.8700), 274.0(1.1225), 228.0(1.5974), 206.0(1.5222)。 Anal. for C12H13NO6 (267.23) calcd :C,54.22 (53.93); H,5.21( 4.90); N,5.43( 5.24).
2.7 Synthesis of Schiff base(VI)
3-methoxy-4-carboxylmethoxybenzaldehyde(1g, 5mmol) was dissolved in 60% ethanol, and 2-amino-4-methylphenol (0.615g,5mmol) was added. The pH of the mixture was adjusted to 7~8 by triethylamine. The reaction mixture was stirred at 50°C~60°C for 6h under nitrogen. After cooled the precipitate was filtered off and washed three respectively with anhydrous ethanol and anhydrous ether to give yellow powder(0.99g, 62.7%), mp 178.2°C~178.6°C. 1H NMR(CDCl3) d:13.85(s,1H,-COOH),13.11(s,1H,-OH), 8.75(s,1H,-CH=N),7.85~6.80 (m,6H,Ar-H), 4.85(s,2H,OCH2-CO), 3.95(s,3H,OCH3),2.29(s,3H,Ar-CH3)ppm; 13C NMR(CDCl3): 169.0 (-COOH),157.1 (CH=N),150.8,150.2,150.0,136.3,130.8,128.7,128.3,123.8,117.1,114.8, 113.4, 110.9(Ar), 65.3 (-OCH2CO-), 55.5(-OCH3),19.8(-CH3).IR(KBr)n:3787(O-H), 3115(w,COOH), 1650(C=N), 1599,1556, 1516(Ar,C=C), 1375(-CH3), 1271,1240(C-O-C)cm-1; UV (lmax/nm)(Abs): 354 (0.8631), 316.0(0.8532), 280.0(0.9313), 208.0(2.4077)。Anal. for C17H17NO5 (315.32) calcd :C, 65.02 (64.75); H,5.56( 5.43); N,4.15( 4.44). 3. RESULTS AND DISCUSSION
3.1 Synthesis of Schiff bases or intermediates
Synthesis of intermediates: Excellent conditions of preparing intermediates(I), the mole ratio of vanillin to sodium hydroxide and 1-bromobutane was 1:1:2. Reaction temperature was usually 50°C~60°C. Excellent condi- tions of preparing intermediates(II), the mole ratio of vanillin to sodium hydroxide and 1-bromobutane was 1:2:1.1.
Optimum factore of synthesis of Schiff bases: The reaction conditions were different to synthesis of these Schiff bases. A large number of experiments showed that the best reaction times for preparation Schiff base(III) was 13h; for Schiff base(IV), 32h; for Schiff base(V), 48h; for Schiff base(VI) 6h.At the same time, the pH of synthesis of Schiff bases was controlled from 7 to 8.
3.2 UV-Vis spectroscopy
The intermediates or Schiff bases were dissolved in anhydrous ethanol respectively to give thinning solution(~10-5mol·L-1). The reference liquid was anhydrous ethanol in all survey. Their UV spectroscopies were show as the follows.
The important bands of Schiff base(III)is given at 354.0. The band at 354nm, is n-p* absorption spectrum(weak), and the conjugate structure of C=N and benzene cycle. On the other hand, the conjugate takes form between the oxyalkyl groups and benzene cycle. So, the band is red shift. But Schiff base(IV)is given at 278.0. It is n-p* absorption spectrum(weak), and is the conjugate structure of C=N and benzene cycle. On the other hand, the ortho-operation of C=N group is –CH2OH, which hinders the conjugate structure between C=N group and benzene cycle, the absorption band of conjugate system results in blue shift, and the absorption strength comes down[24].
The important band of Schiff base(V) is given at 310.0. The band at 310.0nm is n-p* absorption spectra (weak), and is the conjugate structure of C=N and benzene cycle. And the Schiff base(VI) is given at 354.0. It is n-p* absorption spectrum(weak). The band at 280.0nm, which is like other Schiff base, is absorption spectrum of meticulous structure of benzene cycle.
3.3 IR spectroscopy
The various peaks of the intermediate(I) and (II) accord with that reported literature[23].
For the Schiff base(III), the band at 3395cm-1 is stretch absorption spectrum of O-H bond of phenol. The new band at 1626cm-1 is stretch absorption spectrum of C=N bond of the product. The others at 1599cm-1 and 1580cm-1 are stretch absorption spectrum of C=C bonds of aromatic skeleton. They show that the Schiff base(III) is synthesized with 3-methoxy-4-butoxybenzaldehyde and 2-amino-4-methylphenol. The stretch absorption spectrum of C=N bond at 1626cm-1 makes red shift because conjugating with C=N and aromatic cycle[29].
For the Schiff base(IV), the band at 3329 cm-1 is stretch absorption spectrum of O-H bond of alcohol and the band at 1608 cm-1 is stretch absorption spectrum of C=N bond of the product, making red shift because of conjugating with C=N and aromatic cycle19. This suggest that the Schiff base(IV) is synthesized with 3-methoxy-4-butoxybenzaldehyde and 2-aminophenylmethanol.
For the Schiff base(V), the band at 3485cm-1 is wide stretch absorption spectrum of O-H bond of COOH group, which forms hydrogen bond, and the bands at 1648cm-1and 1645 cm-1 are stretch absorption spectra of C=N bond of the Schiff base(V). And the Schiff base(VI), the band at 3787 cm-1 is stretch absorption spectrum of O-H bond of phenol. The band at 1650cm-1 is stretch absorption spectrum of C=N bond of the Schiff base(VI).
3.4 NMR analysis
The chemical shift of proton of phenolic hydroxyl group is 4.5~8.0 in many literatures. But, the proton shifts of phenolic hydroxyl group of Schiff base(III) and (IV) are 13.10 and 13.11 respectively. The obvious downshift was caused by the forming of hydrogen bond[25,26].
The feature absorption bands of C=N group show at 156.9 for Schiff base(III) , at 159.4 for the Schiff base(IV), at 168.7 for the Schiff base(V) and at 157.1 for the Schiff base(VI), respectively. This suggests C=N group of the products. On the other hand, the characteristic absorption band of aromatic C joined oxygen shows 160.0~165.0 in the intermediates and Schiff bases.
Sum up, the results of analysises confirmed the structure of aim compounds. Acknowledgement This research was supported grants from the Yulin Teacher's college and Guangxi Department of education (Guangxi Education Researches[2003]No.22). The authors thank characterization center of Guangxi University for IR spectra, and characterization center of Guangxi Normal University for elements analysis and NMR spectra.
REFERENCES
[1] Hu W X, Sun Y Q, Yuan Q, Yang Z Y. Chemical Journal of Chinese Universities, 2002,
23: 1877(in Chinese).
[2] Hiroaki N, Takeshi F, Tokenori H, Isao M, Tashiyuki A, Shiro M and Toru M. J. Med.
Chem, 1974, 17(12): 1312.
[3] Csedes J, Mulles W, Tosh W. J. Antibiotics, 1983, 36(8):1020.
[4] Sauser J Ed, Boulton A J, Oxford: Elsevior, 1996, 6: 901.
[5] Chen H S, Li Z M, Li J F. Chemical Journal of Chinese Universities, 2000,20:1520(in
Chinese).
[6] Palacios F, Retana A M O, Pagalday J. Tetrahedron, 1999,55:14451.
[7] Xu L, Duan T H, Li M H. Zhongguo Yiyao Zazhi, 1996,30(1):41(in Chinese).
[8] Jin G Y, Cao C Y, Li L T, Ren J, Zhao G F. NongyaoxueXuebao, 1999,1(1):15(in Chinese).
[9] Alxander V, Chem. Rev, 1995,95:273.
[10] Ali S M, Iftikhark Ahmad N. Synth. React. Inorg. Met.-Org. Chem., 1983,13(6):699.
[11] Pandeya S N, Sriram D, Nath G, De Clercq E. Europ Pharm. Sci, 1999,(9):25.
[12] Pandeya S N, Sriram D, Nath G, De Clercq E. Pharm. Acta Helv., 1999,74:11.
[13] Bradshaw J, Asay R E, Mass G E, Izatl R M, Christensen JJ. J. Heterocyclic Chem.,
1978,15:825.
[14] Zhu W R, Hu P Z, Li M Y, Huang X L, Wu C T. WUHAN UNIVERSITY JOURNAL OF NATURAL
SCIENCES,2003,8(2A):433.
[15] Li M Y, Hu P Z, Zhu W R and Wang Y. Asian Journal of Chemistry,2003,15(1):38.
[16] Li M Y, Hu P Z, Zhu W R, Xu K X. Chinese Chemical Letters,2003,14(6):572.
[17] Zhu W R, Hu P Z, Li M Y, Huang X L, Wu C T. J. Analytical Science,2003,19(4): 333.
[18] Zhu W R, Hu P Z, Li M Y, Wu C T, Huang X L. Chin. J. Org.Chem.,2004,24(3):346(in
Chinese).
[19] Qiao Y H, Sun M, Wang J L, Miao F M. Journal of Tianjin Light Industry College, 2002,
(4): 8(in Chinese).
[20] Sun M, Duan Y Q, Wang J L, Miao F M, Liu X L, Chemical Journal of Chinese
Universities, 2001,22(7):1160(in Chinese).
[21] Li X Q, Yan Z H, Wu Z Z, Le Z F, Liu X Q, Wan F Z. Journal of Central China Normal
University(Natural Sciences), 1994,28(3): 345(in
Chinese).
[22] Huang L, Yu D Q. Ultraviolet Spectra Applied in Organic Chemistry (First Vol),Science
Press,Beijing, 2000:120.
[23] Deng Q Y, Liu L, Deng H M,Spectroscopy in Organic Chemistry (First), Science
Press,Beijing, 2003:49.
[24] Deng Q Y, Liu L, Deng H M, Spectroscopy in Organic Chemistry (First), Science
Press,Beijing, 2003:21.
[25] Zeng Q D, Qian M, Gou S H, Fun H K, Duan C Y, You X Z. Inorg.Chim. Acta,1999,294:1.
[26] Balsells J, Mejorado L, Phillips M, et al. Tetrahedron:Asymitry,1998,9:4135.
[27] Elwahy A H M. Tetrahedron, 2000,56:897.
朱万仁, 黄志伟,李家贵,陈渊, 晏全,胡培植#,吴成泰#
(玉林师范学院化学与生物系,中国玉林,537000; #武汉大学化学与分子科学学院,中国武汉,430072)
摘要 本文报道以香兰素和1-溴丁烷以及氯乙酸合成了两个中间体。然后,个别与2-氨基-4-甲基苯酚、2-氨基苯甲醇、甘氨酸反应制备了四种新型席夫碱。对中间体和席夫碱产物均进行了元素分析、UV、IR、1HNMR和13CNMR表征。
关键词 香兰素,席夫碱,合成