http://www.chemistrymag.org/cji/2007/094019pe.htm |
Apr. 2,
2007 Vol.9 No.4 P.19 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding, 071002)
Received Jan. 28, 2007.
Abstract Under the
assistance of the efficient transfer-reaction between the dithiocarbonate group and
radicals in RAFT polymerization, the graft
copolymerization of methyl acrylate (MA) onto chitosan with
high grafting efficiency was performed. The effects of polymerization conditions, such as initiator concentration, monomer concentration, reaction
time and temperature, on grafting parameters were studied. The graft copolymer was
confirmed by a series of characteristic techniques including Fourier transform infrared
spectroscopy (FTIR), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). The
experimental results have shown that the introduction of the dithiocarbonate groups onto
chitosan has efficiently increased the graft efficiency, via minimizing the formation of
homo-polymers in graft copolymerization.
Keywords Chitosan; Graft copolymerization; Reversible addition-fragmentation chain
transfer (RAFT) polymeization
1. INTRODUCTION
Chitosan, N-deacetylated derivative of chitin, is extensively studied in recent years,
because it is biodegradable, biocompatible, nontoxic cheap and abundant in nature.
However, as it is well known, chitosan is a type of semi-crystal and compact macromolecule
whose insolubility in water limits its application.
Fortunately, the structure of chitosan contains a large number of reactive groups such as
hydroxyl and amino groups; it can thus be modified by various chemical reactions [1-4]. A lot of researches were concentrated on the
chitosan modification through copolymerization of chitosan and vinyl monomers for the
preparation of new materials, which can endow chitosan with special properties and
versatile applications [5]. Conventionally,
vinyl monomers are grafted onto chitosan using various induction methods such as redox
systems, radiation, microwave and so on, to conduct the polymerization [6-8].
Graft efficiency is the most important parameter in graft copolymerization, howeverฃฌit is not mentioned or in a low
level in most of the previous literatures [9-14]. For example,
chitosan-g-polystyrene initiated with 60Co gamma radiation and chitosan-g-polyacrylamide
induced by microwave were once studied, but neither of the two literatures displayed the
graft efficiency and another microwave induced reaction produced chitosan-g-poly
(methylmethacrylate) with its highest graft efficiency of 40%[10-12].
Reversible addition-fragmentation chain transfer polymerization (RAFT),
the youngest living polymerization, which appears to be the most versatile
controlled radical polymerization with respect to the monomer and the reaction medium,
leading to the development of novel polymeric materials. In
RAFT polymerization, effective RAFT agents for this process are dithioesters [Z-C (S=)
S-R] with various leaving R-groups and stabilizing Z-moieties that involves
dithiobenzoates, dithioalkanoates, trithiocarbonates, dithiocarbonates (xanthates) and
dithiocarbamates[15]. The basic working principle of RAFT polymerization is
that the reversible addition-fragmentation process of thiocarbonyl-thio groups in RAFT
polymerization can minimize the relative rate of bimolecular termination, and keep most of
the growing polymeric chains bonding to the thiocarbonyl-thio compound and still remaining
potentially active throughout the polymerization process [16-17]. On the other
hand, in the presence of conventional initiator, initial and macromolecular radicals with
different molecular weight (homo-polymers) were produced and some of them easily combined
with dithiocarbonyl groups. With time going on the reversible addition-fragmentation
actions at last create a polymer including dithiocarbonyl moieties. These characteristics
implied that if the RAFT agent moiety is first introduced into the macromolecules, such as
chitosan, it might facilely create initiating sites and further synthesize the graft
copolymer of chitosan and common vinyl monomers with higher graft efficiency.
In this study, we first
introduced the dithiocarbonate groups, the reversible addition-fragmentation chain
transfer moiety, onto chitosan via the reaction of amino group with benzyl chloride and carbon
disulfide. And then, this modified chitosan was directly applied to produce chitosan-g-polymethylacrylate
(chitosan-g-PMA) with higher grafting efficiency in the presence of common
initiator. In addition, effects of the initiator concentration, temperature, time, amount of
water, and ratio of monomer to modified chitosan on the grafting copolymerization were
investigated by determining the grafting parameters, i.e. grafting efficiency, grafting
percentage and monomer conversion. It was found that the grafting efficiency was
considerably enhanced under the assistance of radical transferring interaction of
dithiocarbonate groups on chitosan, comparing with the conventional grafting methods.
2. EXPERIMENTAL
2.1 Materials
Chitosan (deacetylation degree > 85%, Mn=4.6กม105) used in this study was
prepared from indigenous waste shrimp shell [18]. Methyl acrylate (MA) was
washed successively with aqueous sodium hydroxide and distilled water to remove the
inhibitors, and distilled finally. The grafting initiator, potassium persulfate (KPS) was
recrystallized from water. Other chemicals were of analytical grade and were used without
further treatment.
2.2 Measurements
Fourier transform infrared measurements were used to confirm the structure of
chitosan-g-PMA with a Bruker EQUINOX55 (Bruker, Germany) in the potassium bromide pellets.
The X-ray diffraction analysis was measured with a Yaa 900 X-ray diffraction instrument
(Dandong Ray Apparatus Corp., China). The TGA curves were completed with a Pyris6 DTATG
apparatus (PerkinElmer, America) in atmospheric N2 at a heating rate of 10กใC/min.
2.3 Graft copolymerization of MA onto modified chitosan
A glass tube containing a magnetic bar, deionized water, KPS and modified chitosan
prepared according to the literature [19] was first pretreated with standard
cycles of evacuation with nitrogen gas and then charged with required amount of monomer by
a syringe. The reaction mixture was thermostatically controlled at
the required temperature and magnetically stirred. After the completion of the reaction,
the reaction mixture was cooled and poured into methanol. The precipitated product was filtered through a weighted,
sintered glass funnel and was washed several times with methanol. Then, the crude graft
copolymer was dried to a constant weight in vacuum at 50กใC. The homo-polymer of
PMA was removed from the crude graft copolymer by exhaustive Soxhlet extraction with
acetone for 48 h. The final graft copolymer was dried under vacuum at 50กใC to a constant weight.
Grafting parameters
used in this study were defined and calculated as follows:
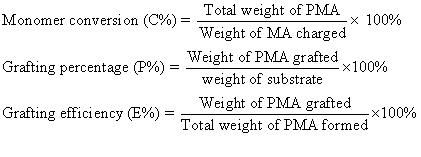
3. RESULTS AND DISCUSSION
3.1 Formation of graft copolymer
The graft polymerization of MA onto modified chitosan under the assistance of
dithiocarbonate moiety was shown in Scheme 1. Some initial radicals from KPS initiate
homo-polymerization of MA, while another great part of them bond to the chitosan by the
transfer-reaction with dithiocarbonate moiety to produce new radicals. In the same way,
the macroradicals of homo-PMA formed in the system is apt to anchoring onto chitosan
backbone, producing the titled graft copolymer. It is worthwhile to state that, similar to
the conventional grafting method, a part of hydroxyl and amino groups can also be
converted to macroradicals through the direct transfer-reaction of KPS radicals, and
leading to the initiation of monomer MA and chain-grow of PMA. In the experiment for
comparison, the grafting efficiency is 40.2% and 67.7% for pure chitosan and modified
chitosan used as matrix, implying that introducing dithiocarbonate moiety onto chitosan
have great effect on increasing the graft efficiency.
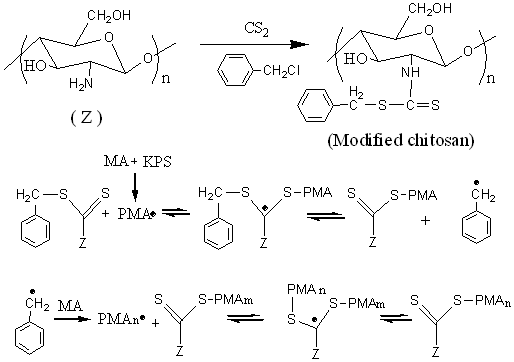
Scheme 1. A proposed mechanism for the graft
polymerization
3.2 Effects of reaction condition on grafting
parameters
3.2.1 Effect of initiator concentration
3.2. 2 Effect of reaction temperature
The effect of temperature on grafting parameters was studied by changing the reaction temperature from 55กใC to 70กใC and keeping the other reaction condition constant. It can be seen from Fig. 2 that with the rise of temperature, E% kept decreasing; and C % increased in the whole process. The P % reached maximum value 133% at 60 กใC and then decreased thereafter. This may be ascribed to the following reasons that the radicals in the reaction system become much reactive due to the increase of temperature, and the radical-transfer reaction to other molecules including monomer, solvent and homo-polymer of MA was also accelerated, resulting in the formation of more and more homo-polymer of MA and increase of E %. It can be concluded that lower temperature is beneficial to obtain the chitosan-g-PMA with high grafting efficiency.
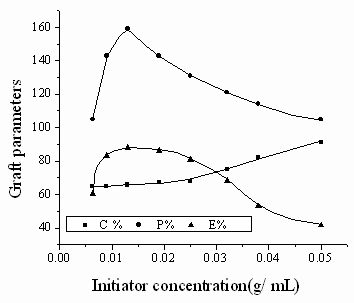
Fig. 1 Effect of KPS concentration on graft parameters
Chitosan: 0.15g; MA: 0.42mL; water: 1.0mL; time: 1h; temperature: 60 กใC
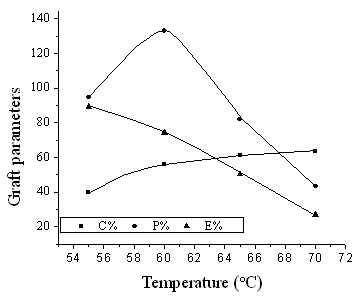
Fig. 2 Effect of reaction temperature on graft parameters
Chitosan: 0.15g; MA: 0.42mL; water: 1.0mL; KPS: 0.013g; time: 1h.
3.2.3 Effect of water
When the other conditions were invariable (MA: 0.42mL and chitosan: 0.15g), the effect of
water amount on graft parameters is shown in Fig.3. It was found that E % was slightly
affected by the amount of water, and reached its maximum value 78.4%, when water was
1.0mL. P % and C % decreased in the whole process due to the dramatically lowering
radicals concentration in water phase as water increased. According to the curve of E %,
it was concluded that when the amount of water was at lower level relative to chitosan,
the reaction system could not be dispersed effectively, therefore the probability of
chitosan contacting with PKS radicals decreased. Furthermore, excessive water has the same
effect because of the reduction of initiator concentration.
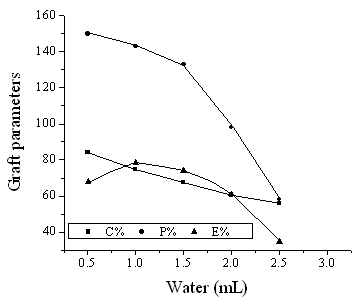
Fig. 3 Effect of water on graft parameters
Chitosan: 0.15g; MA: 0.42mL; KPS: 0.013g; temperature: 60กใC; time: 1h.
3.2.4 Effect of reaction time
Fig. 4
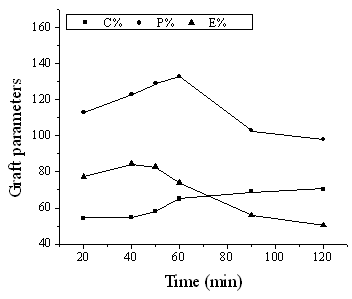
Fig. 4 Effect of reaction time on graft parameters
Chitosan: 0.15g; MA: 0.42mL; water: 1.0mL; KPS: 0.013g; temperature: 60 กใC.
3.2.5 Effect of monomer
concentration
As shown in Fig. 5, with the increase in ratio of MA, P % increased remarkably, and C %
went up slightly and then kept almost flat. Besides, there was not a significant changing about E %. This behavior of E % could be explained by the fact that
an increase of monomer concentration had no direct effect on the radical concentration as
well as other reaction condition. In fact, the most radicals in the reaction mixture only
existed in water phase and on the surface of chitosan.
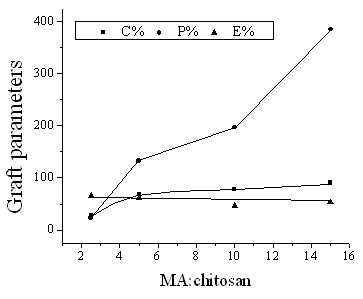
Fig. 5 Effect of monomer
concentration on graft parameters
Chitosan: 0.15g; temperature: 60กใC; water:
1.0mL; KPS: 0.013g; time: 1h.
3.3 Characterization of chitosan-g-PMA
3.3. 1 FTIR spectroscopy
Fig. 6 depicts the FTIR spectra of chitosan,
modified chitosan and chitosan-g-PMA with graft percentage of
197%. The FTIR spectrum of chitosan shows some characteristic absorption bands of chitosan
at about 3437 cm-1 (nN-H and vO-H) 1635 (
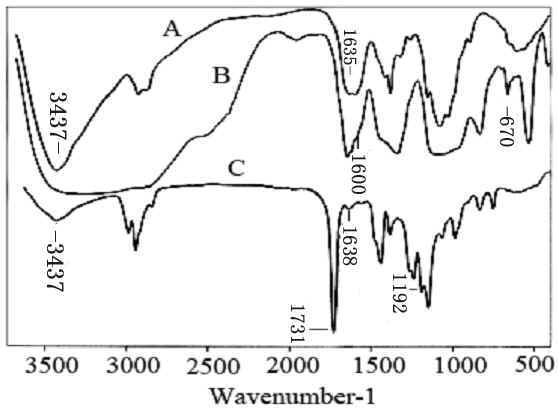
Fig. 6 FTIR spectra of chitosan (A), modified chitosan (B) and chitosan-g-PMA (C)
3.3.2 XRD analysis
The graft copolymerization of MA onto chitosan was also supported by XRD measurement of
pure chitosan and chitosan-g-PMA (G%=197%). As shown in Fig.7, The chitosan-g-PMA
with high grafting percentage exhibited a single broad absorption at 2q = 21.5o, appeared to be due
to extensive chain coverage of PMA, because of the lacks complete stereoregularity of PMA
macromolecular chains. Contrast to this, two major peaks at about 10o and 20o
ascribed to 020 and 110 reflections, respectively, were observed in the XRD pattern of
pure chitosan with more crystallinity. The result elucidates that the crystalline
structure was changed to some extent owing to introduction of PMA onto chitosan
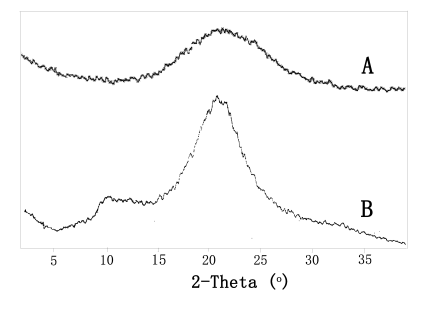
Fig. 7 XRD patterns of chitosan-g-PMA
(A) and chitosan (B)
3.3.3 Thermograms of the grafted
chitosan
Fig.8 displays the thermograms for graft
copolymer (G %=197%), modified chitosan and chitosan. Both chitosan and modified chitosan
showed a weight loss with two stages under heating in an inert atmosphere. The first
weight-losing stage spanned over 55 กใC to 230 กใC and exhibited about
5% loss in weight, which was ascribed to the loss of adsorbed and bond water. The second
stage of weight loss started at 230 กใC and continued up to
500 กใC, due to the degradation of chitosan and modified
chitosan. Different from chitosan and modified chitosan, chitosan-g-PMA
demonstrated only one-stage degradation without the weight loss related to adsorbed and
bond water. This can be explained by the following reason that hydrophobic property of
graft copolymer was considerably increased duo to the attachment of hydrophobic PMA onto
chitosan surface. Chitosan-g-PMA showed a higher thermostability with weight loss
of 20% at about 350 กใC as compared to chitosan and modified chitosan, which
displayed about 35% and 50% weight loss respectively. As an accessorial proof, the
remarkable difference in thermal properties among chitosan, modified chitosan and
chitosan-g-PMA illustrated that PMA chains has been grafted onto chitosan surface.
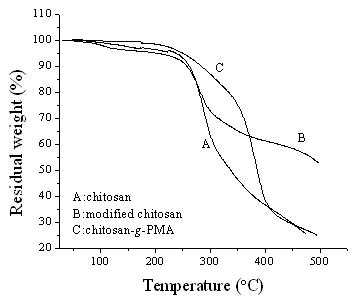
Fig. 8 Thermogravimetric trace of graft
copolymer, modified chitosan and chitosan
4. CONCLUSIONS
In order to increase the grafting efficiency, a new method of grafting MA onto
chitosan by aiding of RAFT agent has been developed in this work. The effect of various
experimental conditions on the grafting parameters has been systematically evaluated and
the FTIR, TGA and XRD measurements elucidated that methyl acrylate was efficiently grafted
onto chitosan surface. The highest grafting efficiency in this study is found to be about
90% due to introducing dithiocarbonate groups onto chitosan under this condition-chitosan: 0.15g; KPS concentration: 0.013g/mL; temperature: 55 กใC; reaction time: 1 hour.
REFERENCE
[1] Huang M F, Fang Y E, Biopolymers, 2006, 82: 597.
[2] Chen T H, Kumar G, Harris M. T. et al. Biotechnology and bioengineering, 2000, 70:
564.
[3] Yu H J, Wang W S, Chen X S et al. Biopolymers, 2006, 83: 233.
[4] J. Fangkangwanwong, Mitsuru Akashi, Toshiyuki Kida et al. Biopolymers, 2006, 82: 580.
[5] Kang H. -M. Cai Y-L. Liu P-S Carbohydrate Research, 2006, 341(17), 11: 2851.
[6] E. Yilmaz , T. Adali , O. Yilmaz, Reactive & Functional Polymers 2007, 67: 10.
[7] Wang J P, Chen Y Zh, Ge X W et al. Chemosphere 2007, 66: 1752.
[8] Ge H C, Pang W, Luo D K, Carbohydrate Polymers 2006, 66: 372.
[9] Yao P J, Wan H Y, Jiang L B et al. Technology&Development of Chemical
Industry,2005, 34: 9.
[10] Vandana Singh, Ashutosh Tiwari, Devendra Narayan Tripathi et al Polymer 2006, 47:
254.
[11] Vandana Singh, Devendra Narayan Tripathi, et al, Carbohydrate Polymers 2006, 65: 35.
[12] Liu P F, Zhai M L, Wu J L, Radiation Physics and Chemistry, 2001, 61:149.
[13] Zhang J, Yuan Y L, Shen J et al. European Polymer Journal 2003, 39: 847.
[14] Cao L Y, HuangJ F, Cao J J et al. Speciality Petrochemicals, 2001, 3: 19.
[15] Hideharu Mori, Motonobu Matsuyama, Kazuhiko Sutoh et al. Macromolecules 2006, 39:
4315.
[16] Michelle L. Coote, David J. Henry, Macromolecules 2005, 38: 5774.
[17] Christine Schilli, Michael G. et al. Macromolecules 2002, 35: 6819.
[18] Pourjavadi A, Mahdavinia GR, Zohuriaan-Mehr MJ et al. J Appl Polym Sci 2003; 88:
2048.
[19] Tao L R, Mei G Z, Ming CH T et al. Chemical Journal of Chinese Universities, 1999,
20: 1897.