http://www.chemistrymag.org/cji/2007/095021pe.htm |
May 2,
2007 Vol.9 No.5 P.21 Copyright |
(1 Department of Chemistry, Jinan University, Guangzhou 510632; 2 Institute of Nanochemistry, Jinan University, Guangzhou 510632; 3Department of Biotechnology, Jinan University, Guangzhou 510632, China)
Received on Mar.12, 2007; Supported by Foundation of Ministry of Education (No.2002247), Foundation of Jinan University (No.640071) and Foundation of Guangdong province (No.2003C33101).
Abstract Liquid–liquid equilibrium tie line data were determined for two ternary systems of water + dimethoxymethane + diisopropyl ether or 2,2,4-trimethylpentane at 298.15 K and ambient pressure. The experimental liquid–liquid equilibrium results have been successfully correlated by a modified and an extended UNIQUAC models both with binary and ternary parameters.Keywords Liquid–liquid equilibria, Octane boosters, Ternary mixtures, Modified and extended UNIQUAC models
1. INTRODUCTION
Recently several fuel oxygenates have
been added to gasoline to enhance the octane number and reducing air pollution. So, these
reformulated gasolines contain a large amount of octane boosters such as methyl tert-butyl
ether (MTBE), diisopropyl ether (DIPE) and dimethoxymethane (DMM). 2,2,4-Trimethylpentane
(TMP) is an essential component in gasoline. As a part of our research work on
thermodynamic properties of octane boosters [1,2], this paper reports liquid–liquid equilibrium (LLE)
measurements on two ternary systems water + DMM + DIPE or TPM at 298.15 K. The
experimental LLE data were correlated by means of the modified UNIQUAC and extended
UNIQUAC models [3,4] including both binary and ternary parameters. The binary
parameters of miscible binary mixtures of constituents of the ternary systems were
obtained from vapor–liquid
equilibrium (VLE) data [5,6] and those of immiscible mixtures were obtained
from mutual solubility data [7,8].
2. EXPERIMENTS
2.1 Materials
TMP was supplied by the Tianjin Damao Chemical Reagents Factory, with minimum mass
fraction of 0.990. DIPE was provided by the Tianjin Kermel Chemical Reagents Development
Center, with a mass fraction of 0.995 at the fewest. DMM was provided by the Shanghai
Chemical Reagents Company, Inc., with minimum mass fraction of 0.995. All chemicals were
used without further purification. For GC analysis, appreciable peaks have not been
detected. Water was distilled twice.
2.2 Apparatus and Procedures
Ternary LLE measurements were carried out at 298.15
± 0.01 K. About 70 cm3 of ternary mixtures were poured into the equilibrium
glass cell placed in a thermostated water bath. The mixture was then stirred vigorously by
magnetic stirrer for 3 h and then allowed to settle for 3 h, which was sufficient to
separate into two liquid phases. Dry nitrogen gas was used to prevent contamination from
moisture in the headspace of the equilibrium cell. Samples, withdrawn from upper and lower
phases in the cell by a microsyringe, were analyzed by a gas chromatograph (GC-14C)
equipped with a thermal conductivity detector. Each component of the ternary mixtures was
separated clearly, using a stainless steel column (2 m long, 3 mm i.d.) packed with
Porapak QS. The temperatures of the injection system and detector system were all set at
513.15 K. The initial temperature and final temperature of the oven was kept at 483.15 K
and 453.15 K, respectively. The hydrogen flow rates for both the separation and reference
columns were set at 1.1 cm3 s-1. The
peak areas of the components, detected with a chromatopac (N2000), were calibrated with
prepared known mixtures by mass. The mass of each component of the mixture was determined
from the calibration and converted to mole fraction. Three analyses were done for each
sample to obtain a mean value with a reproducibility of less than 0.1% deviation. The
accuracy of the measurements was estimated within ±0.001 in mole fraction.
2.3 Experimental results
Tables 1 and 2 show experimental LLE data for the water + DMM + DIPE and water + DMM +TMP
mixtures.
Table 1 Equilibrium phase compositions in mole fraction for the ternary mixtures of water (1) + dimethoxymethane (2) + diisopropyl ether (3) at 298.15 K
Organic phase |
Aqueous phase |
||||
|
|
|
|
|
|
0.0455 |
0.2229 |
0.7316 |
0.9819 |
0.0169 |
0.0012 |
0.0616 |
0.2924 |
0.6460 |
0.9779 |
0.0210 |
0.0011 |
0.0650 |
0.3735 |
0.5615 |
0.9706 |
0.0280 |
0.0014 |
0.0575 |
0.4334 |
0.5091 |
0.9639 |
0.0352 |
0.0009 |
0.0635 |
0.4778 |
0.4587 |
0.9600 |
0.0386 |
0.0014 |
0.0875 |
0.5072 |
0.4053 |
0.9550 |
0.0434 |
0.0016 |
0.0898 |
0.5440 |
0.3662 |
0.9535 |
0.0457 |
0.0008 |
0.0703 |
0.5819 |
0.3478 |
0.9527 |
0.0462 |
0.0011 |
0.0624 |
0.6205 |
0.3171 |
0.9492 |
0.0496 |
0.0012 |
0.0848 |
0.6326 |
0.2826 |
0.9446 |
0.0542 |
0.0012 |
Table2 Equilibrium phase compositions in mole fraction for the ternary mixtures of water (1) + dimethoxymethane (2) + 2,2,4-trimethylpentane (3) at 298.15 K
Organic phase |
Aqueous phase |
||||
|
|
|
|
|
|
0.0129 |
0.1374 |
0.8497 |
0.9852 |
0.0148 |
0.0000 |
0.0298 |
0.2344 |
0.7358 |
0.9814 |
0.0173 |
0.0013 |
0.0539 |
0.2935 |
0.6526 |
0.9700 |
0.0300 |
0.0000 |
0.0658 |
0.3460 |
0.5882 |
0.9654 |
0.0346 |
0.0000 |
0.0729 |
0.4426 |
0.4845 |
0.9563 |
0.0437 |
0.0000 |
0.0474 |
0.5195 |
0.4331 |
0.9514 |
0.0486 |
0.0000 |
0.0481 |
0.5535 |
0.3984 |
0.9474 |
0.0526 |
0.0000 |
0.0705 |
0.5828 |
0.3467 |
0.9452 |
0.0548 |
0.0000 |
0.0669 |
0.6052 |
0.3279 |
0.9416 |
0.0584 |
0.0000 |
0.0502 |
0.6339 |
0.3159 |
0.9408 |
0.0592 |
0.0000 |
0.0582 |
0.6724 |
0.2694 |
0.9378 |
0.0622 |
0.0000 |
3. CALCULATION PROCEDURE AND RESULTS
3.1 Calculation procedure
We have used the modified UNIQUAC[3] and extended UNIQUAC[4] models with binary and ternary parameters for an accurate
description of the experimental ternary LLE data as well as binary VLE and mutual
solubility data.
The binary parameter ![]() defined by the binary energy parameter aji is expressed as
defined by the binary energy parameter aji is expressed as
![]() (1)
(1)
where aji can be obtained from binary experimental phase equilibrium
data, and C was set to 1 for the extended UNIQUAC and 0.65 for the modified
UNIQUAC.
The binary energy parameters for the miscible mixtures were obtained
from the VLE data reduction using the following thermodynamic equations[9]:
![]() (2)
(2)
![]() (3)
(3)
where P, x, y, and? are the total pressure, the liquid-phase mole
fraction, the vapor-phase mole fraction, and the activity coefficient, respectively. The
pure component vapor pressure, ![]() , was
calculated by using the Antoine equation with coefficients taken from the literatures[10,11]. The liquid molar volume,
, was
calculated by using the Antoine equation with coefficients taken from the literatures[10,11]. The liquid molar volume, ![]() , was obtained by a modified Rackett equation[12]. The fugacity coefficient, F, was
calculated by the Eqn.(3). The pure and cross second virial coefficients, B, were
estimated by the method of Hayden and O'Connell[13]. The binary energy parameters for the partially miscible
mixtures were obtained by solving the following thermodynamic equations simultaneously.
, was obtained by a modified Rackett equation[12]. The fugacity coefficient, F, was
calculated by the Eqn.(3). The pure and cross second virial coefficients, B, were
estimated by the method of Hayden and O'Connell[13]. The binary energy parameters for the partially miscible
mixtures were obtained by solving the following thermodynamic equations simultaneously.
![]() (4)
(4)
![]() and
and ![]() ( I, II = two liquid phases ) (5)
( I, II = two liquid phases ) (5)
The ternary LLE calculations were carried out using the Eqns.(4) and (5). The
additional ternary parameter τijk was obtained by fitting the model to
the ternary experimental LLE data and the ternary parameterτijk was determined from the ternary
experimental LLE data using a simplex method[14] by minimizing the objective
function:
![]() =
=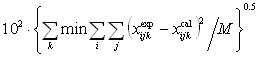 (6)
(6)
where min means minimum values, i = 1 to 3 for ternary mixtures, j = phases I
and II, k = 1,2,…,n (no. of tie lines), M = 2ni, and x = (the
liquid-phase mole fraction).
3.2 Calculation results
Table 3 presents the constituent binary energy parameters of the modified and extended
UNIQUAC models. Table 4 shows the ternary parameters obtained in fitting the modified and
extended UNIQUAC models to the experimental ternary LLE systems, and root-mean-square
deviation of the mole fraction of tie lines between the experimental and calculated
results for the ternary LLE systems. It seems that the extended UNIQUAC model with the
only binary parameters predicts the ternary LLEs more successfully than the modified
UNIQUAC model, and these models can give a much more accurate representation for the
ternary LLEs by including the ternary parameters in addition to the binary ones. Figures 1
and 2 give the comparison of the experimental and correlated liquid–liquid equilibrium results of two ternary systems of
(water + DMM + DIPE or TMP) as show type 2 at T = 298.15 K. DMM can dissolve in TMP
with any proportion. So addition of DMM will not bring separation in gasoline. Figures 1
and 2 show good agreement between the experimental values and those correlated using
additional ternary parameters. Both models can describe accurately the ternary
experimental LLE data by the correlation involving the additional ternary parameters.
Table 3 Calculated results of binary phase equilibrium data reduction
System (1+2) |
T /K |
No. of data points |
Model |
Energy parameters |
Ref. |
|
a12/K |
a21/K |
|||||
DMM + DIPE |
314.85~334.45 |
9 |
Ia |
–188.41 |
415.98 |
[5] |
DMM + TMP |
314.55~352.75 |
10 |
I |
169.97 |
57.20 |
[6] |
DIPE + water |
298.15 |
MSc |
I |
1590.60 |
166.68 |
[7] |
DMM + water |
298.15 |
MS |
I |
1691.10 |
60.11 |
This work |
TMP + water |
298.15 |
MS |
I |
3023.30 |
1145.60 |
[8] |
a
Modified UNIQUAC model; b Extended UNIQUAC model; c Mutual solubility.Table 4 Calculated results for ternary liquid-liquid equilibrium at 298.15 K
ternary parameters |
deviations f |
||||||
system (1+2+3) |
no.a |
model |
τ231 |
τ132 |
τ123 |
predd |
corre |
| water + DMM + DIPE | 10 |
Ib |
0.0463 |
–0.2659 |
0.1818 |
0.97 |
0.93 |
IIc |
–0.0017 |
0.0123 |
0.0125 |
0.60 |
0.60 |
||
water + DMM + TMP |
11 |
I |
0.1091 |
0.3774 |
0.0999 |
2.40 |
1.15 |
II |
0.3715 |
–3.1506 |
0.5169 |
1.77 |
0.74 |
||
a
Number of tie lines.b Modified UNIQUAC model
c Extended UNIQUAC model.
d Predicted results using only binary parameters.
e Correlated results using binary and ternary parameters.
f Root-mean-square deviation (mol%).
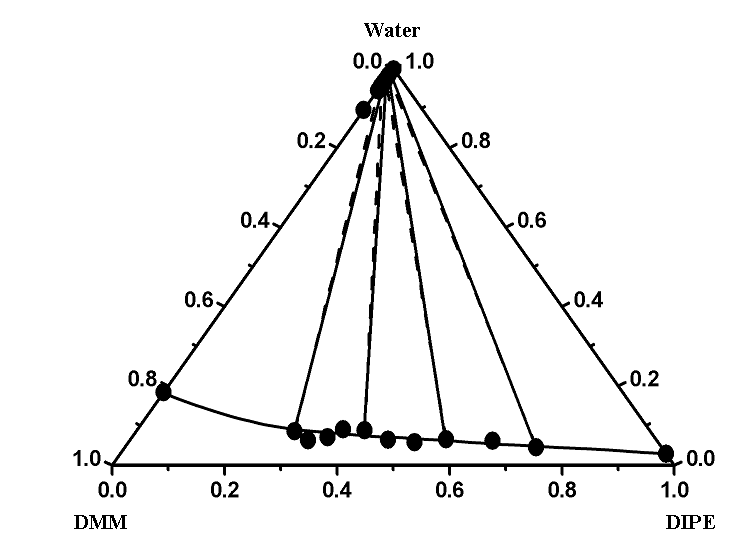
Figure 1 Experimental and calculated (liquid + liquid) equilibria of the ternary mixtures of (water + DMM + DIPE) at T = 298.15 K. ●- - -●, Experimental tie line; ——, correlated by the extended UNIQUAC model with binary and ternary parameters taken from Table 4.
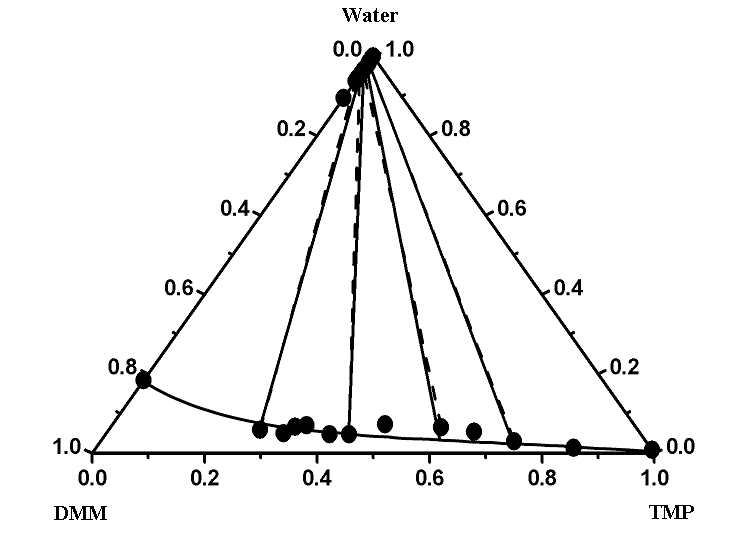
Figure 2 Experimental and calculated (liquid + liquid) equilibria of the ternary mixtures of (water + DMM + TMP) at T = 298.15 K. ●- - -●, Experimental tie line; ——, correlated by the extended UNIQUAC model with binary and ternary parameters taken from Table 4.
4. CONCLUSION
The ternary liquid–liquid equilibrium of the water + DMM + DIPE and water +
DMM + TMP systems were measured at 298.15 K in this work. The experimental ternary liquid–liquid equilibrium data were
successfully correlated by using both models including binary and ternary parameters. The
ternary liquid–liquid
equilibrium results calculated by the extended UNIQUAC model are more suitable agreement
with the experimental results.
REFERENCES
[1] Dong Y H, Chen Y, Pan Z J, et al. J. Jinan Univ Nat Sci Med Edu
(China), 2006, 27: 87–91.
[2] Peschke Nicole, Sander I S. J Chem Eng Data, 1995, 40: 315–320.
[3] Tamura K, Chen Y, Yamada T et al. J Solution Chem, 2000, 29(5): 463–488.
[4] Nagata I. Fluid Phase Equilibria, 1990, 54: 191–206.
[5] Suresh Reddy K V N, Prasad Dasika H L, Abburi Krishnaiah. J Chem Eng Data, 2004, 49:
1546–1549.
[6] Suresh Reddy K V N, Prasad D H L, Krishnaiah A. Fluid Phase Equilibria, 2005, 230: 105–108.
[7] Arce A, Marchiaro A, Rodri'guez O, et al. J Chem Eng Data, 2002, 47, 529–532.
[8] J. M. Sørensen, W. Arlt. Liquid-Liquid Equilibrium Data Collection, Vol. V,
Part 1, DECHEMA, Frankfurt/Main, 1979.
[9] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculation for Multicomponent
Vapor-Liquid and Liquid-Liquid Equilibria . Prentice-Hall: Englewood Cliffs, NJ, 1980:19.
[10] Riddick J A, Bunger W B, Sakano T K. Organic Solvents; 4th ed.; Wiley-Interscience:
New York, 1986.
[11] Reddy S K V N, Prasad H L D, Krishnaiah A. J Chem Eng Data, 2004, 49: 1546–1549.
[12] Spencer C F, Danner R P. J Chem Eng Data, 1972, 17: 236–241.
[13] Hayden J G, O'Connell J P. J Ind Eng Chem Process Des Dev, 1975, 14: 209–216.
[14] Nelder J A, Mead R. J Computer, 1965, 7: 308–313.
陈瑶1,2*, 张胜利1,2,李任强3
(1 暨南大学化学系;2 暨南大学纳米化学研究所,3 暨南大学生物工程系,广州 510632)
2007年3月12日收稿。国家教育部留学回国人员科研基金(No.2002247), 广州暨南大学科研基金(No.640071) 和广东省科技计划基金(No.2003C33101)。
摘要 测定了两个三元体系水、二甲氧基甲烷、二异丙醚和水、二甲氧基甲烷、异辛烷在298.15K和常压下的液液相平衡数据,并用含有二元和三元参数的modified UNIQUAC 和 extended UNIQUAC 热力学模型关联了这些实验数据,两个模型的计算结果均和实验结果较吻合。
关键词 液液平衡,含氧化合物,三元混合物,Modified和extended UNIQUAC 热力学模型