http://www.chemistrymag.org/cji/2007/095020pe.htm |
May 2,
2007 Vol.9 No.5 P.20 Copyright |
(School of Material Science & Engineering, Shaanxi University of Science & Technology, Xi'an 710021, China)
Received Feb.22, 2007.
Abstract Ultrafine
substituted M-type barium ferrite BaTiCoFe10O19 powders were
synthesized successfully by sol-gel method. The hydroxide precursor particles were
formed in gel solution containing ethanol and water at a ratio of 1:1 and NH4OH
as precipitation agent. The effects of molten salt Na2CO3 on
formation of BaTiCoFe10O19 nano-powders were studied. The precursors
were calcined at 900oC for 2h. XRD analysis indicated single phase substituted
M-type barium ferrites were formed with and without molten salt. Molten salt Na2CO3
at 5-15M% resulted in obviously decrease of particle size of powders, which
decreased with increasing amount of molten salt. The particle sizes of 32.2-48.5nm closing
to the theoretical single-domain size and good crystallinity of particles ensure perfect
microwave absorbing properties for the substituted M-type barium ferrites. SEM analysis
indicated that powders were platelike particles with smaller aspect ratio in morphology
which were most suitable for application of microwave absorption materials.
Keyword sol-gel method, preheat treatment, molten salt,BaTiCoFe10O19, powder
1. INTRODUCTION
Substituted M-typed barium hexaferrite Ba(TiCo)xFe10-2xO19 exhibits
a high saturation magnetization and a high coercivity because of the relatively high
magnetocrystalline anisotropy field[1] and it has been explored for many
technological demanding and challenging application, such as for high-density type
perpendicular recording media and microwave materials[2-4]. The properties of
Ti-Co-substituted barium ferrite are largely dependent on the processing routes used for
fabrication, multidomain ferrite, increase in magnetic and absorbing properties as
particle size decrease and crystallinity increase. Therefore, the preparation of a barium
ferrite powder with refined particle size, narrow particle-size distribution, minimal
particle agglomeration, and high crystallinity have been received considerable attentions.
Many processing routes have been devised, including hydrothermal process[5]
microemulsion technique[6] et al.
Sol-gel method have the advantages of low cost and simple technological
process and is an useful method to prepare ultrafine Co-Ti-substituted Ba-ferrite powders
with single-domain perfect crystallography, narrow size distribution and excellent
magnetic properties and perfect absorbing ability. Many technological factors influence
the character of powders prepared with these processing routes. In which, molten-salt
method was effective for decreasing the particle size and agglomeration of powders,
preheat treatment have been verified to be effective in decreasing particle size of
powders. The objectives of the present works are to study the effects of molten-salt and
preheat treatment on the formation and the character of the Co-Ti-substituted M-type
Ba-ferrite powders.
2.1 Preparation of hydroxide Precursor
The ferric chloride hexahydrate and barium chloride dihydrate and cobalt chlorite hexahydrate were dissolved in the solution containing ethanol and water at a ratio of 1:1. Ttitanium propoxide was dissolved in anhydrous ethanol and stabilized with a little acetylacetone. Then two solutions were mixed and stirred for 0.5h. In the mixed solution, the molar concentration were 0.005M for Ba2+, Co2+, and Ti2+ cations, respectively, and was 0.05M for Fe3+ cation. Then, ammonia water was dropwisely slowly added into the resultant solutions at room temperature and constant stirring condition until pH>9. After the precipitation was completed, the precipitate slurry was filtrated, washed with anhydrous ethanol until its pH≈7. As-washed precursor was divided in to four parts. Anhydrous sodium carbonate at the content of 0M%, 5M%, 10M%, and 15M% were added respectively with stirring and were dried at 95oC for 2-5h and put in chamber respectively.
2.2 Heat Treatment and powder characterization
Three portions of as-dried precursor containing 0N% Na2CO3 was preheated at 250oC, 300oC and 350 oC for 0.5--1h respectively. All the precursors were heated at a heating rate of 20oC/min and calcined for 2h at 900oC in air, respectively. The cooling was performed at a slow rate in furnace. The calcined powders were soaked in water at 80oC for 4h and rinsed repeatedly to wash off NaCl and then dried at 95oC for 1h. The phase identification of the calcined Ti-Co-substituted Ba- ferrite powders were conducted at room temperature using X-Ray diffractometer (XRD, CuKα1, l=0.15406nm, Model No. D/Max-2200PC, Rigaku, Japan). The phase and particle sizes of powders were determined with the Jade5 analysis software carried with X-Ray diffractometer. The particles morphology and particles agglomeration of the powder were analyzed using scanning electron microscopy (SEM, Model No: JXM-6700F, Japan).
3. RESULTS AND DISCUSSION
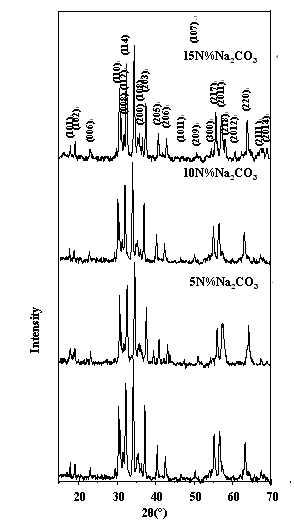
Figure 1 XRD patterns of BaTiCoFe10O19 powders with different amouts of molten salt added To investigate the effects of molten salt on the particle size and agglomeration of powders, molten salt NaCO3 at the contents of 0N%, 5N%, 10N%, and 15N% were added to hydroxides, respectively. As-added precursors were dried and calcined at 900oC for 2h. The XRD patterns of the powders were illustrated in Figure 1, which indicated that BaTiCoFe10O19 is the only detectable phase. Particle sizes determined with strong peak(114) at 2q≈34.12o were 48.5nm, 37.6nm, 34.3nm, and 32.2nm for NaCO3 content of 0M%, 5M%, 10M%, and 15M% respectively, as shown in Figure 2, which indicate that molten salt NaCO3 can remarkably decrease the particle size of BaTiCoFe10O19 powders and particle size decreased as increasing molten salt content.
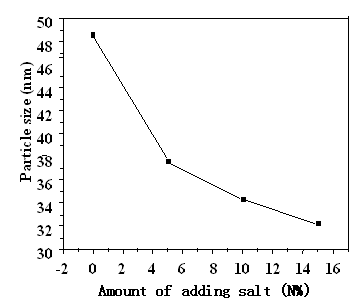
Figure 2 The relation of particle size of BaTiCoFe10O19 powders with amount of molten salt
The SEM photographs were shown in Figure 3 and indicated that particles of powder were hexagonal platelets with 20-40nm of thickness and 20-300nm of width. The average particle size determined with average value of width and thickness was in a range of 20-130nm that decreased with the increase in NaCO3 content. The agglomerations of powders were less. These indicated that molten salt method is effective in reducing the agglomeration and particle size of powders. The effects of molten salt in the decrease of particle size can be contributed to inhibition of NaCO3 on the growth of BaTiCoFe10O19 crystallite. The effect of molten salt in decrease of agglomeration can be contributed to the separation effect of washing away NaCO3 between particles.
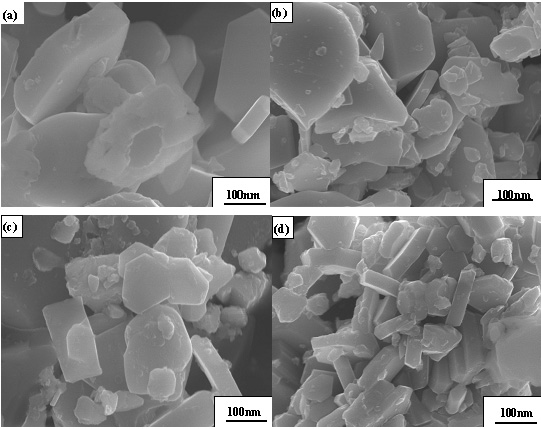
Figure 3 SEM photographs of BaTiCoFe10O19 powders with (a) 0N%, (b) 5N%, (c) 10N% and (d) 15N% molten salt NaCO3
To investigate the
effect of preheat treatment on the formation of powders, the hydroxide precursors without
adding molten salt were preheated at 250oC, 300oC, 350oC
for 50min respectively, followed by calcined at 900oC for 2h. As-calcined
powders were characterized with XRD and SEM at room temperature respectively. The XRD
patterns of the powders as shown in Figure 4 indicated that BaTiCoFe10O19
is the only detectable phase and the particle sizes of powders were 37.6nm, 36.5nm and
39.9nm for preheat temperature of 250oC, 300oC and 350oC
respectively as shown in Figure 5. Compared with particle size (48.5nm) of powder without
preheat treatment, preheat treatment also result in decrease in particle size of BaTiCoFe10O19
powders. The saturation density of nucleation is higher and the size of nucleation
is smaller for the lower temperature of nucleation. Thus the preheat treatment results in
the formation of larger number and smaller size of g-Fe2O3 crystallite, which reacted with BaO,
TiO2 and CoO at high temperature and induce the formation of larger number and
smaller size of BaTiCoFe10O19 particles. However, the particle size
at preheat temperature of 250oC was larger than that of 300oC, which
may be due to stable g-Fe2O3
nucleation was not formed at lower temperature of 250oC. The SEM
photograph of BaTiCoFe10O19 powder preheated at 300oC for
50min as shown in Figure 6 indicated that the particles were hexagonal platelets with
15-40nm of thickness and 15-250nm of width and 15-120nm of average particle size and
agglomeration was less.
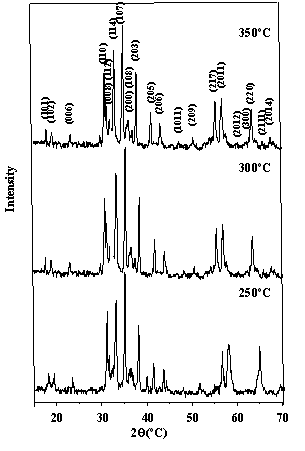
Figure 4 XRD patterns of
BaTiCoFe10O19 powders preheated at different temperatures
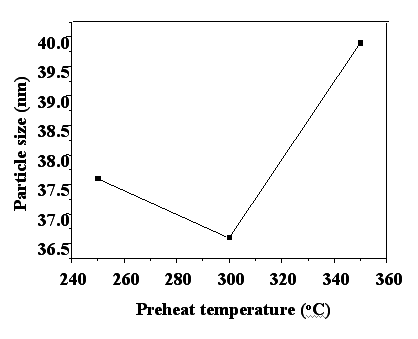
Figure 5 Relation of particle size of BaTiCoFe10O19 powders
with preheat treatment temperature
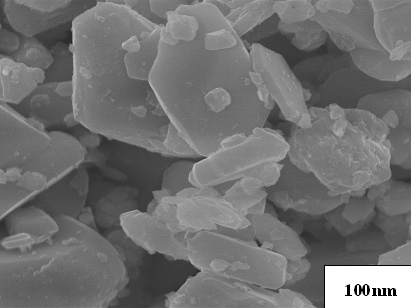
Figure 6 SEM photograph of BaTiCoFe10O19 powder preheated at 300oC
4. CONCLUSIONUltrafine Ti-Co-substituted Ba-ferrite powders have been successfully synthesized with sol-gel method. Molten salt and preheat treatment can remarkably decreased the particle size of powders. The agglomeration of powders can be decreased by washing the powders after calcining. Acknowledgements This work was supported by Natural Science Foundation of Shaanxi University of Science and Technology under the grant No:ZX05-18 REFERENCES
[1] Li Yinyuan, Li Guodong. Physics of ferrite, Beijing, Science Press, 1978, 325.
[2] Bursik J, Simis Z, Stichauer L et al. J. Magn. Magn. Mat., 1996, 157-158(2), 311-312.
[3] Willias J M, Adetunji J, Gregori M. J. Magn. Magn. Mat., 2000, 220(2), 124-128.
[4] Kreisel J, Vincent H, Taeest F. J. Magn. magn. Mat., 2001, 224, 17.
[5] D'Arrigo Maria Cristina, Leonelli Cristina, Pellacani Gian Carlo. J. Am. Ceram. Soc., 1998, 81(11), 3041.
[6] Pankov V. J. Mat. Sci. Eng., 1997, A224, 101. Sol-gel法替代M-型BaTCoFe10O19粉体的制备
沈清
(陕西科技大学材料学院712081)
摘要 应用sol-gel法成功地合成了超细替代M-型BaTiCoFe10O19磁粉。先驱体氢氧化物在醇:水比=1:1的溶胶溶液中以共沉淀法制得。研究了熔盐Na2CO3和预热处理对粉体的粒子尺寸和粘连度的影响。X-射线衍射分析(XRD)分析表明添加5-15N%的Na2CO3的分别在900oC烧成2h后得到了纯的BaTiCoFe10O19粉体。粉体的粒子尺寸随熔盐Na2CO3的添加量增大而减小。250-350oC预热处理也使粉体的粒子尺寸显著减小。SEM分析表明粉体为六角片状,粒子尺寸与XRD分析结果一致。
关键词 sol-gel法,工艺,BaTiCoFe10O19粉体,粒子尺寸