http://www.chemistrymag.org/cji/2007/099040pe.htm |
Sep.4,
2007 Vol.9 No.9 P.40 Copyright |
Preparation and application of novel fluorescein covalently attached to silica nanoparticles for ultrasensitive immunoassays
a
Deng Yingchun, bMeng Jianxin , aNing Qiaoyu, a Li Yuejuan, cHuang Zi, d Liu Xiaoyan(aDepartment of Chemistry, bInstitute of Nano-Chemistry, cDepartment of Biology, dNo.1 Affiliated Hospital, Jinan University, Guangzhou 510632, China)
In an effort to prepare efficient and uniform
bio-label, novel fluorophore doped silica nanoparticles via water-in-oil (W/O)
micro-emulsion method have been developed. The embedded fluorophore was connected to the
backbone of the silica nanosphere via reaction among 6-aminofluorescein,
3-aminopropyltriethyloxysilane and diethylenetriaminepentaacetic dianhydride. The
nanoparticles showed several unique advantages in simply preparation, good photo-stability
and significantly reduced dye leaching in solution. A fluoroimmunoassay for detecting
trace level human IgG was developed with the present fluorescein doped silica nanosphere,
The calibration graph for human IgG was linear over the range of 0.1-225ng/mL with a
detection limit of 0.06 ng/mL. It was shown that the present fluorescein doped nanosphere
are high-quality labels for biochemical assays.
Keywords Aminofluorescein; Microemulsion; Silica nanosphere; Human IgG; Fluoroimmunoassay
Recently, fluorescent doped nanoparticles have been used extensively for a wide range of applications in biological detection and diagnosis. As a novel fluorescence material, the nanoparticles have the advantages in small and uniform size, high hydrophilic, strong fluorescence, high stability and good biological compatibility. Silica nanosphere doped with various fluorophore, such as dye [1] or lanthanide chelate [2-3], and silica coated nanostructure such as Ag/SiO2 [4-5], Au/SiO2 [6], magnetite/SiO2 [7-10], CdS/SiO2 [11], have been studied. Sensitivity of the fluorescence assay using fluorophore doped nanosphere usually have been greatly enhanced compared with that using small fluorophore molecule, since large amount of fluorophore molecules could be doped in single nanoparticle. Doping of fluorophore in silica may also enhance the fluorescence efficiency by isolating the fluorophore from surrounding water molecules, as water molecule often causes significantly solvent fluorescence quenching. In addition, silica has been demonstrated to be an excellent substrate suitable for many surface immobilization mechanisms. The silica surface also aids in the dispersion of nanoparticles in aqueous solution, thus it is highly "soluble" in aqueous solution. These properties make silica nanopartilces suitable for application in solution-based bioassays. However, in most cases, fluorophore was merely physically dispersed in cavities of the network structure of silica nanoparticles. This type of physically embedding is somewhat unstable and fluorophore leaching is often unavoided. In a previous work by Duan [12], the dye leaching of fluorescein doped silica nanoparticles has been studied. They proved that the dye leaching could be greatly reduced by attaching fluorescein to a Y-type protein. A new type of fluorescein embeded silica nanoparticles was proposed in the present work. Unlike the physically embedding in Duan’s work, the embedded fluorescein was chemically covalent by attached to the backbone of the silica nanoparticles via reaction among aminofluorescein, 3-aminopropyltriethyloxysilane and diethylenetriaminepentaacetic dianhydride. The consequent fluorescein embedded mono-dispersed silica nanosphere show satisfactory photo-bleaching and dye leaching properties. The application of the present fluorescein doped silica nanoparticles in immunoassay was demonstrated with a sandwich-model immunoassay for the detection of IgG in human serum and satisfactory result was achieved. 2. EXPERIMENTAL
2.1. Materials and Instruments
6-Aminofluorescein (6-AMF, Acros), 3-aminopropyltriethyloxysilane (APTEOS, Acros), tetraethyl orthosilicate(TEOS, Aldrich) were used as received. Goat anti-human IgG antibody (ghIgG) and human IgG were supplied by Beijing Dingguo biotechnology Co. The patient serum was supplied by the No.1 affiliated hospital of Jinan university. Magnetite nanoparticles were prepared with reference [13] method. Phosphate-buffered saline (PBS) were prepared by mixing 8.3mM Na2HPO4, 137mM NaCl, 1.7mM KH2PO4, 3.0mM KCl, 0.05%(v/v) Tween-20, together, pH=7.4. Tris-HCl were prepared by mixing 50mM tris(hydroxymethyl)aminomethane, 70mM hydrochloric acid(25%), 0.05%(v/v) Tween-20, together, pH=7.8. All other chemicals including dimethylsulfoxide (DMSO), Triton X-100, n-hexanol, cyclohexane, ammonium hydroxide (28-30%, mass), glutaraldehyde (25% water solution), diethylenetriaminepentaacetic dianhydride (DTPAA) were of analytical purity and used without purification. Twice distilled water was used throughout.
The fluorescence spectra were recorded with a Hitachi F-4500 fluorescence spectrophotometer. Transmission electron microscopy (TEM) images of the particles were obtained from Philip Tecnai-10 transmission electron microscope.
2.2. Methods
2.2.1. Synthesis of monomer fluorophore silica precursor
AMF-DTPAA-APTEOS monomer fluorophore silica precursor (ADA) was prepared by the following procedure: DTPAA (0.14mmol) was dissolved in 2mL DMSO, then 6-AMF (0.14mmol) was added with stirring, 33mL APTEOS (0.14mmol) was added after stirring for 10h. The process of the reaction was monitored at ambient temperature overnight. The resultant yellow mixture was directly used in the following procedure without further purification.
2.2.2. Synthesis of fluorescein covalent doped nanoparticles (ADA nanoparticles)
A W/O reverse microemulsion was prepared with nonionic surfactant Triton X-100 using the procedure similar to that described by Ye[14] at ambient temperature, microemulsion containing 2.3mL Triton X-100, 2.3mL n-hexanol, 9.3mL cyclohexane, 200mL TEOS, 1.1mL of water and 200mL ADA was mixed with another microemulsion which containing 2.3mL Triton X-100, 2.3mL n-hexanol, 9.3mL cyclohexane, 200mL ammonium hydroxide with vigorous stirring. The reaction was allowed to continue for 24h to ensure completion of the condensation, then the nanoparticles were isolated by acetone, followed by centrifuging and washing with ethanol and water several times to remove any surfactant and un-reacted materials. The particles were then vacuum dried for over 20h at 30° C to get the ADA nanoparticles.
2.2.3 Photo-bleaching experiments
10mg ADA nanoparicles was dispersed in 4mL water in a 1cm quartz cuvette. After sealed with PARA-film to avoid vaporization of solvent, the cuvette was exposed to a deuterium lamp. Fluorescence was determined after exposure for every 5 min or more. A water solution of ADA was put in another quartz cuvette, sealed and determined fluorescence for comparison.
2.2.4. Dye leaching experiments
10mg ADA nanoparticles were added in 4mL water followed by ultrasonic for 15min to obtain a suspension. Fluorescence intensity of the suspension was determined. Then the suspension was centrifuged and the precipitation was re-dispersed in 4mL water again. The leaching extent of fluorescent material could be understood from the change of fluorescence intensity with repeating of the dispersion and centrifuge processes. Similar experiment was done for comparison with a fluorescein doped nanoparticles in which fluorescein was not covalent by attached to the silica network.
2.2.5. Chemical modification on the nanoparticle surface to immobilization antibody
Amino-functionalized ADA nanoparticles were prepared on the basis of that method [15] by Yang. ADA nanoparticles were re-dispersed in micelles containing 4.6mL Triton X-100, 4.6mL n-hexanol, 18.6mL cyclohexane, 2.2mL water, then 100mL TEOS and 60mL of ammonium hydroxide (25%) were added. 80mL of APTEOS was introduced into the microemulsion after 30 min. The reaction was allowed to continue for 24 h at ambient temperature. The purification course is the same as in 2.2.2. Amino groups introduced through APTEOS modification make it possible for covalent immobilization of various biomolecules onto the surface of ADA nanoparticles via reaction with glutaraldehyde. Immobilization of ghIgG was conducted with the procedure described by Yang et al [15]: 5mg amino-functionalized ADA nanoparticles were dispersed in 3mL PBS containing 5% glutaraldehyde for about 1h. Those nanoparticles were collected by centrifugation and re-dispersed in 3mL PBS, and then incubated with 1mL 100mg.mL-1 ghIgG for 12h at 4° C with stirring. The ghIgG-modified ADA nanoparticles (ADA-ghIgG) were washed with PBS several times to remove excessive antibody and kept at 4°C in PBS.
2.2.6. Covalent immobilization of antibody onto magnetite nanoparticles
Amino-functionalized magnetite nanoparticles were prepared in this way: 0.75mg magnetite nanoparticles were dispersed in a solution of 50mL water and 50mL ethanol, after 15min ultrasonic treatment, 4mL APTEOS was added, the reaction was performed at 60° C for 10h with stirring, then the particles were magnetite separated and washed with ethanol and distilled water until there is no oleaginous suspension, dried for use. The preparation of ghIgG antibody immobilized magnetite nanoparticles (Fe3O4-ghIgG) is similar to the procedure for ADA-ghIgG as described in 2.2.5 unless the amino-functionalized magnetite nanoparticles were used instead of the amino-functionalized ADA nanoparticles.
2.2.7. Human IgG capture (sandwich) assays
The immunoassay procedure for human IgG using ADA nanoparticles as the label was as follows: To a tube 100mL 1.67mg.mL-1 of Fe3O4-ghIgG PBS solution prepared as described in 2.2.5 was added, then added different amount of satandard human IgG solution or properly diluted patient serum. The reaction mixture was incubated for 1h at 37° C, magnetite separated and washed twice with PBS and once with Tris-HCl. Then 80mL 1.67mg.mL-1 ADA-ghIgG PBS solution was added and incubated in 1mL PBS buffer for 2 h at 37° C. The mixture was magnetite separated again and washed twice with PBS and once with Tris-HCl. The precipitation was re-dispersed in 3mL PBS buffer to determine the fluorescence property. The IgG concentration in the patient serum sample was analyzed in the hospital laboratory with the conventional ELISA method for comparison.
3. RESULTS AND DISCUSSION
3.1 Synthesis of ADA
The ADA was synthesized via the reaction as shown in Scheme 1. The reaction mixture, which may certainly contain by-products like AMF-DTPAA-AMF and APTEOS-DTPAA-APTEOS, was directly used in successively procedure for synthesis of the ADA nanoparticles without any purification. The reason for ignoring the purification process lies in the facts that these by-products can be easily removed in the procedure when washing the ADA nanoparticles with water and ethanol, and the manipulation for extracting ADA from the reaction mixture were rather complicated.
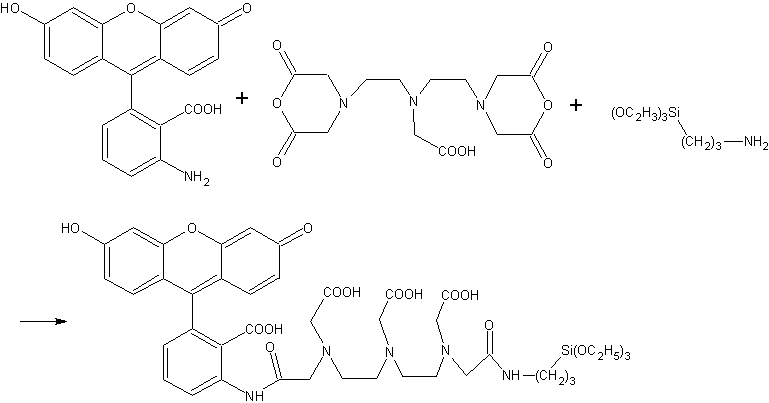
Scheme 1 Synthesis of the monomer fluorophore silica precursor 3.2 Preparation of ADA nanoparticles or amino-functionalized ADA nanoparticles
The microemulsion route has been found to be an easy and efficient method for the preparation of such nanoparticles. Various amount of ADA monomer precursor were used in the preparation of ADA nanoparticles and a recommended volume of 200mL was concluded to obtain both the strongest fluorescence and perfect shapes of silica nanoparticles. The size of the nanoparticles was primarily controlled by the molar ratio of water to surfactant and the molar ratio of water to TEOS. The TEM image (Fig. 1) showed the ADA nanoparticles were very uniform with a typical diameter of 60±5nm. The surface availability of primary amine groups on the amino-functionalized nanoparticles varied with the adding time and amount of APTEOS and the aging time of polymerization.
|
Fig. 1 TEM image of ADA nanoparticles |
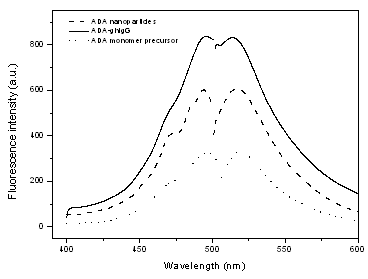 |
Fig.2 Spectra of ADA monomer precursor, ADA nanoparticles and ADA-ghIgG |
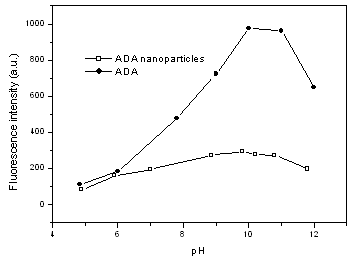
3.3 Effect of pH on fluorescence of ADA
nanoparticles
The ADA nanoparticles show similar fluorescence
spectrum with fluorescein as shown in Fig.2. Both ADA nanoparticles and ADA show an
emission at 521 nm when excited at 494 nm. This is not surprising since the shape of the
spectrum of fluorescein is insensitive to solution environment. However, fluorescence
intensity of fluorescein is very sensitive to pH of water solution, while that of ADA
nanoparticles is much more inert as shown in Fig. 3: both ADA and ADA nanoparticles give
the strongest emission at pH 9-10, but the emission intensity of ADA nanoparticles at pH 9
is about 3.5 times of that at pH 5, much less than the value of 8.6 for ADA. This
difference is believed to be a result of the embedding of fluorescein into silica
nanoparticles: most of the doped fluorescein was inside the nanosphere instead of on the
surface and was well shielded from the solution environment. The shielding effect of
silica nanoparticles is not complete and fluorescence of ADA nanoparticles can still be
somewhat affected by pH. Two reasons may response to the fact: first, small parts of the
doped fluorescein were on the surface of the nanoparticles and contact directly with the
water environment, second, water molecules or other ions in the solution may still
penetrate into the small cavity in these nanoparticles which formed from silica, a rather
hydrophilic compound.
|
Fig.3 Effect of pH on fluorescence intensity |
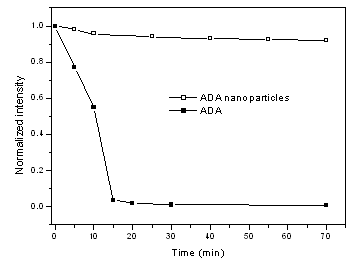
It was known that fluorescence of fluorescein was bleached when exposed to violet light. Similar results were obtained from our photo-bleaching experiments on ADA solution as shown in Fig. 4: the fluorescence intensity dropped sharply to 0.9% of the initial intensity after 15 min exposure. However, photo-bleaching observed for ADA nanoparticles in solution is very small and was even neglectable after short time exposure (Fig. 4): The fluorescence intensity for ADA nanoparticles decreased to 96% of the initial intensity in 20 min exposure and keep almost constant after that. These results suggested that the ADA nanoparticles are very photo-stable. It is believed that the increase of photo-stability for ADA nanoparticles is attributed to two factors: first, immobilization of fluorophore in silica network restraint the movement of fluorophore molecules; Second, the silica coating protects against the penetration of dissolved oxygen into the silica network. Minim of photo-bleaching of ADA nanoparticles in the initial period of exposure may be the contribution of the fluorophore distributed on the surface of the nanoparticles.
|
Fig.4 Results of photobleaching experiments |
3.5 Dye leaching experiment
A main problem for the fluorophore doped silica nanoparticles is the unstable property
caused by dye leaching when exposed to an aqueous environment for biological applications.
The present ADA nanoparticles showed satisfactory results in dye leaching experiment as
shown in Fig. 5. After washing 11 times with water, the fluorescence intensity of ADA
nanoparticles remained 78.9% of the initial intensity, compared with 8.6% for that of
fluorescein non-covalent doped silica nanoparticles. This difference indicated that the
fluorophore in ADA nanoparticles has been firmly covalent attached to the silica framework
through DTPAA and APTEOS, which prevent the fluorophore leaking from silica, Duan[12]
has proposed another strategy to prevent dye leaching in fluorescein doped silica
nanoparticles, in which fluorescein was bonded to IgG, a Y-shape protein, which can
increase the steric hindrance and protected the dye leaching from the nanoparticles.
However, IgG is prone to change its shape after denaturalization, which may cause addition
leakage of the dye. The large protein volume also prevents high doping concentration of
fluorescein into the silica nanoparticles.
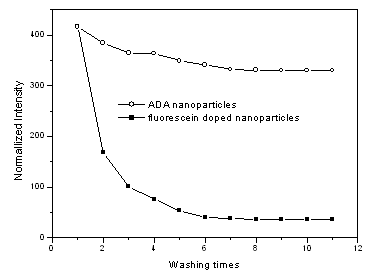 |
Fig.5 Stability ADA nanoparticles in water solution |
3.6 Human IgG capture assays in a sandwich format
Attachment of ghIgG to the ADA nanoparticles did not affect the fluorescence spectrum as shown in Fig. 2. With the present ADA nanoparticles, human IgG capture assays were successfully completed in a sandwich format as described in 2.2.7. Good linear relation was observed between the fluorescence intensity of ADA nanoparticles and human IgG concentration over the range of 0.1-225ng.mL-1 as shown in Fig. 6. The linear calibration equation F=2.7783-9.9909c was obtained. The detection limit of the method is 0.06ng.mL-1 given by the calculation with equation LOD=3s/s, compared with the results by W. Yang et al[15] using FHS silica nanoparticle probe to detected HbsAg, in which the linear range is 0.5–220 ng.mL-1 with a detection limit of 0.1 ng.mL-1, The accuracy and reliability of the present method was investigated by performing the recovery tests. The recovery was obtained according to the general procedure shown in Table 1. The recovery experiments gave satisfactory results of 86.3% with a 3.8% relative standard deviation on the means of three determinations.
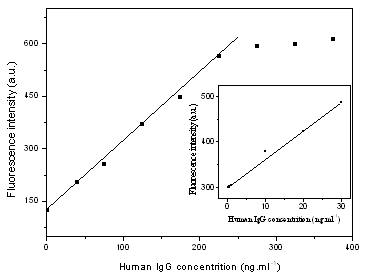 |
Fig.6 Calibration curve of human IgG determination |
Table 1 Analytical results of patient serum sample
Nominal concentration of patient serum IgG |
Found Concentration of patient serum IgG |
Standard adding of human IgG |
Total amount found |
Recovery |
80 |
75 |
100 |
161 |
86 |
Fluorescein covalently doped silica nanoparticles for sensitive immunoassays has been prepared with a rapid and inexpensive method. By reaction among 6-amino fluorescein, APTEOS and DTPAA, followed by a water-in-oil (W/O) microemulsion, fluorescein can be covalently attached to the backbone of silica nanopaticles. Fluorescence property of the obtained ADA nanoparticles is rather stable to long time exposure of violet light and water solution. Human IgG capture assays with the present ADA nanoparticles was also successfully completed in a sandwich format. A satisfactory linear range of 0.1-225ng/mL and detection limit of 0.06ng/mL was achieved. The present approach could be taken as a general method for making fluorophore covalently doped silica nanoparticles. The sole requirement for covalently doping of other fluorophore is an amine group in the fluorophore molecule, which could react with the anhydride groups in DTPAA to form the covalent bond. A terbium chelate covalently doped silica nanoparticles has also been prepared with the present way[2]. It is expected that this kind of nanoparticles as effective biological labels will have more applications in biochemistry research. Acknowledgements This research is supported by the National Natural Science Foundation of China (No. 20475021) and Science Foundation Team Project of Guangdong Province (No. 05200555).
REFERENCES
[2] Ning Q Y , Meng J X, Liu Y L ,J. Rare Earth, 2006, 24(2): 193.
[3] Ye Z Q, Tan M Q, Wang G L, Yuan J L. Talanta, 2005, 65: 206.
[4] Ung T, Liz-Marzan LM, Mulvaney P, Langmiur, 1998, 14 (14): 3740.
[5] Li T, Moon J, Morrone A A, et al. Langmiur,1999, 15(13): 4328.
[6] Liz-Marzan L M, Giersig M, Mulvaney P. Langmuir,1996, 12: 4329.
[7] Perez J A, Quintela M A, Mira J et al. J. Phys. Chem. B. 1997, 101(41): 8045.
[8] Dresco P A, Zaitsev V S, Gambino R J et al. Langmiur, 1999, 15: 1945.
[9] Liu C, Zou B S, Rondinone A J et al. J. Phys. Chem. B., 2000, 104: 1141.
[10] Santra S, Tapec R, Theodoropoulou N et al. Langmuir, 2001, 17: 2900.
[11] Correa-Duarte M A, Giersig M, Liz-Marzan L M. Chem. Phys. Lett., 1998, 286(5-6): 497.
[12] Duan Q H ,Wang K M , Tan W H et al. Chemical Journal of Chinese University (Gaodeng Xuexiao Huaxue Xuebao), 2003, 24(2): 255.
[13] Qu S C, Yang H B, Ren D W et al. Journal of Colloid and Interface Science, 1999, 215: 190.
[14] Ye Z Q, Tan M Q, Wang G L et al. Aanl. Chem., 2004, 76 (3): 513.
[15] Yang W, Zhang C G, Qu H Yet al. Analytica Chimica Acta, 2004, 50(3): 163.
新型的荧光素掺杂二氧化硅纳米粒子用于超灵敏的免疫分析
a邓迎春 b孟建新* a宁巧玉 a李月娟 c黄峙 d刘晓妍
(a暨南大学化学系,b暨南大学纳米化学研究所,c暨南大学生物系,d暨南大学第一附属医院,广州,510632)
摘要 为了制备高效而均一的纳米标记物,我们将6-AFM与DTPAA,APTEOS通过化学键合作用制备荧光单体前驱物,制备了以二氧化硅为外壳大小均一的荧光生物标记物,该方法所制备的纳米粒子光稳定性好,克服了传统方法制备核壳荧光纳米颗粒中存在的荧光染料泄露问题。利用所制得的荧光素纳米粒子对羊抗人抗体IgG进行了标记,该方法在0.1-225 ng/mL之间有较好的线性范围, 最低检出限为0.06ng/mL, 这表明6-ADA纳米粒子是一种很好的生物标记材料。
关键词 氨基荧光素;微乳液;纳米微球;人IgG蛋白;荧光分析。