http://www.chemistrymag.org/cji/2007/098039pe.htm |
Aug.28,
2007 Vol.9 No.8 P.39 Copyright |
Liu Gang, Sun Lin, Liu Chunping, Ji Chunnuan
(School of Chemistry and Materials Science, Ludong University, Yantai 264025, China)
Abstract The novel 6,7,8-trimethoxy
N-substituted-4-aminoquinazoline compounds were synthesized by the reaction of
6,7,8-trimethoxy-4-chloroquinazoline with aryl amines refluxing in i-propanol, starting
from gallic acid that is natural materials. The structures of title products were
characterized by IR, 1H NMR, 13C NMR and elemental analysis.
Meanwhile, the preliminary research for the effects of reaction conditions on
intermediates synthesis was performed.
Keywords quinazoline; gallic acid; synthesis
1. INTRODUCTION
In recent years, quinazoline compounds take on good biological activity in medicine and
pesticide. As part of our ongoing research program on heterocyclic compounds which may
serve as leads in designing novel antitumor agents regarding PD153035
[4-(3-bromophenyl)amino- 6,7-dimethoxyquinazoline] as leading compound, we were
particularly interested in 4-substituted quinazolines[1-3]. We considered the
well known activity of the quinazoline nucleus in chemotherapy, while many of its
substituted derivatives are effective antitumor agents[4-7]. Furthermore, more
recent data have been reported that a broad class of quinazolines also act as potent and
highly selective inhibitors of epidermal growth factor receptor (EGFR) or epidermal growth
factor receptor tyrosine kinase (EGFR-TK)[8-10]; members of this class are
expected to have great therapeutic potential in the treatment of malignant and
nonmalignant epithelial diseases [11,12]. In view of these facts, and in order
to study the influence of 4- and 8-site substituted on antitumor activity, we have
prepared a series of novel 6,7,8-trimethoxy-4-aryl aminoquinazolines starting from gallic
acid that is natural material (Scheme 1), in the hope of discovering more active ATP site
inhibitors.
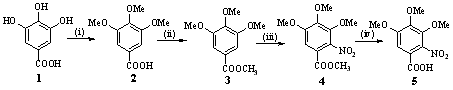
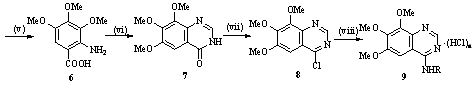
Scheme 1
2. EXPERIMENTAL PROCEDURE
Unless otherwise indicated, all common reagents and solvents were used as obtained from commercial supplies without further purification. All melting points of the products were determined by a XT-4 binocular microscope (Beijing Tech Instrument Co., China) and were not corrected. The infrared spectra were recorded on a Bruker VECTOR22 spectrometer using KBr disks. 1H-NMR and 13C-NMR spectra were performed on a Varian-Inova 500 MHz spectrometer at room temperature with DMSO-d6 as solvent and TMS as internal standard. D2O exchange was used to confirm the assignment of the signals of NH protons. Elemental analysis was performed on an Elementar Vario-III CHN analyzer. The following compounds were prepared as described in the literature: 3,4,5-trimethoxybenzoic acid (2): white solid, yield 81.4%, mp 159-161 ºC (lit. [13], mp 160-162 ºC); methyl 3,4,5-trimethoxybenzoate (3): white needle crystal, yield 69.5%, mp 75-76ºC (lit. [14], mp 82-84 ºC).
2.1 Synthesis of intermediate 4-8
Synthesis of methyl 2-nitro-3,4,5-trimethoxybenzoate (4): To a stirred solution of methyl 3,4,5-trimethoxybenzoate (2.0 g, 8.8 mmol) in AcOH (10 mL), fuming HNO3 (2.5 mL) was added carefully during 30 min at room temperature. After addition of the acid, the solution was allowed to be stirred for 1-2 h. The reaction mixture was then poured into ice water (60 mL). The solid was filtered, washed with water, dried and recrystallized from EtOH-H2O (2:1, V:V). Pale yellow needle crystal, yield 25.4%; mp 62-63ºC. IR (KBr) n: 3013, 2963, 2847, 1719, 1580, 1497, 1545, 1344, 1233, 1113, 866 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 7.34 (s, 1H, PhH), 3.94-3.89 (t, 9H, 3OCH3), 3.83 (s, 3H, COOCH3). 13C NMR (DMSO-d6, 125 MHz) d : 163.3, 154.7, 146.2, 145.5, 139.6, 117.7, 109.2, 63.1, 61.6, 57.1, 53.7. Anal. Calcd for C11H13NO7 (271.2): C, 48.71; H, 4.83; N, 5.16. Found: C, 48.57; H, 5.02; N, 4.91.
Synthesis of 2-nitro-3,4,5-trimethoxybenzoic acid (5): To a stirred solution of methyl 2-nitro-3,4,5-trimethoxybenzoate (14.0 g, 50 mmol) in 0.8 mol/L NaOH (120 mL, 100 mmol) and 95% EtOH (60 mL) at 45-50ºC in water bath for 2 h, the reaction mixture was cooled by ice-water. Then, concentrated hydrochloric acid was added dropwise with stirring while maintaining the temperature at 20ºC. The mixture was adjusted to pH 2-3. The solid was filtered, washed with water, dried and recrystallized from EtOH. Pale yellow needles crystal, yield 92.3%; mp 160-161 ºC. IR (KBr) n: 3107, 2538, 2953, 2851, 1695, 1578, 1497, 1547, 1341, 1246, 1117, 725 cm-1. 1H NMR (DMSO-d6, 500 MHz) d: 7.32 (s, 1H, PhH), 3.93-3.88 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d: 164.1, 154.5, 145.8, 145.3, 139.8, 118.8, 109.3, 63.1, 61.5, 57.0. Anal. Calcd for C10H11NO7 (257.2): C, 46.70; H, 4.31; N, 5.45. Found: C, 46.77; H, 4.14; N, 5.32.
Synthesis of 2-amino-3,4,5-trimethoxybenzoic acid (6): A mixture of powder Sn (5.3 g, 45 mmol) and concentrated hydrochloric acid (18 mL) was stirred for 4-5 h at room temperature. Then, 2-nitro-3,4,5-trimethoxybenzoic acid (2.6 g, 10 mmol) was added to the solution. The reaction mixture was carefully heated to 80ºC in water bath, while maintaining the temperature for 20 min. Then, concentrated hydrochloric acid (5 mL) was added into the reaction mixture, while maintaining the temperature for 10 min. The reaction mixture was cooled by ice-bath and stirred for 10 min. The precipitated complex stannic double salt was filtered, washed with little concentrated hydrochloric acid. The white solid obtained was stirred with portions of 10% K2CO3 solution until it dissolved with pH 9-10 and the mixture was alkaline, whereupon a grayish precipitate appeared and was removed by filter, washed with 10% K2CO3 solution. The solution was neutralized with acetic acid to pH 2-3 while maintaining the temperature below 20ºC. The white solid obtained was filtered, washed with water, dried and recrystallized from EtOH-H2O (1:1, V:V). White needles crystal, yield 61.7%; mp 126-128ºC. IR (KBr) n: 3487, 3379, 3008, 1670, 1576, 1458, 1273, 1149, 744 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 7.07 (s, 1H, PhH), 3.82-3.70 (t, 9H, 3OCH3), 3.45 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz) d : 169.4, 147.6, 142.9, 141.6, 140.4, 1109.3, 104.5, 60.9, 60.6, 56.6. Anal. Calcd for C10H13NO5 (227.2): C, 52.86; H, 5.77; N, 6.16. Found: C, 52.74; H, 5.63; N, 6.02.
Synthesis of 6,7,8-trimethoxyquinazolin-4-one (7): A mixture of 2-amino-3,4,5-trimethoxy benzoic acid (1.1 g, 5 mmol) and excess of formamide (4 mL) was stirred for 5 h at 130-140ºC. After the reaction was over, a suitable water was dropwise to the reaction mixture to resolve the excess of formamide at 100 ºC. The gray solid obtained was filtered, washed with water, dried and recrystallized from EtOH. Pale brown crystal, yield 55.4%; mp 220-222ºC. IR (KBr) n: 3304, 3163, 3009, 1665, 1613, 1474, 1287, 1126, 798 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 12.21 (s, 1H, NH), 8.01 (s, 1H, quinazolone H-2), 7.35 (s, 1H, quinazolone H-5), 3.95-3.88 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 160.6, 152.7, 148.3, 147.7, 143.3, 139.0, 119.2, 101.7, 62.4, 61.4, 56.4. Anal. Calcd for C11H12N2O4 (236.2): C, 55.93; H, 5.12; N, 11.86. Found: C, 56.18; H, 5.18; N, 12.04.
Synthesis of 4-chloro-6,7,8-trimethoxyquinazoline (8): A solution of 6,7,8-trimethoxy quinazlin-4-one (0.6 g, 2.5 mmol) and four drops N,N-dimethylaniline in POCl3 (25 mL) was heated at reflux for 3 h. POCl3 was removed by distillation at reduced pressure and the residue was diluted with 20 mL CHCl3 and treated with 30 g ice-water. The mixture was adjusted to pH 4-5 with saturated potassium carbonate solution. The organic layer was separated and the residue was extracted two times with 40 mL CHCl3, dried by MgSO4, concentrated by distillation, and recrystallized from petroleum ether (b.p. 60-90 ºC). Pale gray solid, yield 62.5%; mp 101-103ºC. IR (KBr) n: 3099, 2945, 2845, 1603, 1468, 1246, 1136, 787 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 8.93 (s, 1H, quinazoline H-2), 7.29 (s, 1H, quinazoline H-5), 4.06-4.01 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.9, 154.3, 150.9, 148.1, 146.3, 142.7, 120.1, 98.6, 62.2, 61.1, 56.3. Anal. Calcd for C11H12N2O4 (236.2): C, 51.88; H, 4.35; N, 11.00. Found: C, 52.02; H, 4.21; N, 10.94.
2.2 Synthesis of 6,7,8-trimethoxy N-substituted-4-aminoquinazoline 9a-9n
A solution of 4-chloro-6,7,8- trimethoxyquinazoline (3.0 mmol) and aryl amines (3.0 mmol) in i-PrOH (30 mL) was stirred under reflux for 4-12 h. Upon completion of the reaction, as monitored by TLC, the solid was filtered, washed with 2-propanol, dried and recrystallized from EtOH-H2O to give the title compounds. Most of it was hydrochloride. If the solid was not appeared while the reaction was completed, the solvent was removed under reduced pressure and the residue was washed with cool water, filtered off and purified by silica gel column chromatography (petroleum ether-ethyl acetate, 5:1 v:v) to give the title compounds. Most of them was free organic-base using this method. Sometimes we can get both hydrochloride compounds and free organic-base compounds in the same reaction.
4-(4-Bromophenyl)amino-6,7,8-trimethoxyquinazoline dihydrochloride (9a). This compound was obtained as pale yellow solid; yield 51.7%, mp170-173ºC. IR (KBr) n: 3011, 2947, 2847, 1628, 1489, 1288, 1128, 804 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.53 (s, 1H, NH), 8.76 (s, 1H, quinazoline H-2), 8.14 (s, 1H, quinazoline H-5), 7.71 (s, 4H, Ph-H), 4.05-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.9, 154.5, 149.6, 148.2, 142.1, 136.5, 132.2, 127.3, 109.9, 100.6, 62.7, 61.9, 57.8. Anal. Calcd for C17H16BrN3O3·2HCl (463.1): C, 44.09; H, 3.92; N, 9.07. Found: C, 43.82; H, 4.18; N, 9.04.
4-(2-Methoxydibenzofuran-3-yl)amino-6,7,8-trimethoxyquinazoline dihydrochloride (9b). This compound was obtained as yellow solid; yield 46.8%, mp160-162ºC. IR (KBr) n: 3011, 2949, 2851, 1626, 1474, 1286, 1125, 764 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.45 (s, 1H, NH), 8.68 (s, 1H, quinazoline H-2), 8.22 (d, J = 7.45 Hz, 1H, dibenzofurane H-5), 8.09 (s, 1H, quinazoline H-5), 8.03 (s, 1H, dibenzofurane H-1), 7.88 (s, 1H, dibenzofura-ne H-4), 7.72 (d, J = 7.45 Hz, 1H, dibenzofurane H-8), 7.58-7.55 (m, 1H, dibenzofurane H-7), 7.47-7.45 (m, 1H, dibenzofurane H-6), 4.04-3.93 (t, 12H, 4OCH3). 13C NMR DMSO-d6, 125 MHz) d : 156.3, 154.0, 150.9, 148.9, 147.7, 145.3, 141.4, 127.7, 123.5, 123.3, 123.1, 121.3, 111.7, 111.4, 108.6, 103.6, 99.8, 62.0, 61.3, 56.9, 56.3. Anal. Calcd for C25H25N3O5·2HCl (520.4): C, 57.70; H, 5.23; N, 8.07. Found: C, 57.60; H, 5.03; N, 8.16.
4-(3-Chlorophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9c). This compound was obtained as pale yellow solid; yield 61.2%, mp 172ºC (dec.). IR (KBr) n: 3013, 2947, 2847, 1628, 1487, 1288, 1126, 785 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.43 (s, 1H, NH), 8.81 (s, 1H, quinazoline H-2), 8.10 (s, 1H, quinazoline H-5), 7.90 (s, 1H, Ph-H-2), 7.71 (d, J = 9.15 Hz, 1H, Ph-H-4), 7.56-7.53 (m, 1H, Ph-H-5), 7.41 (d, J = 9.15 Hz, 1H, Ph-H-6), 4.05-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.4, 153.9, 149.2, 147.6, 138.1, 132.8, 130.4, 126.2, 124.2, 123.0, 109.3, 99.8, 62.0, 61.2, 57.1. Anal. Calcd for C17H16ClN3O3 ·HCl (382.2): C, 53.42; H, 4.48; N, 10.99. Found: C, 53.50; H, 4.57; N, 10.74.
4-(3-Bromophenyl)amino-6,7,8-trimethoxyquinazoline (9d). This compound was obtained as pale yellow solid, yield 19.9%, mp 126-127ºC. IR (KBr) n: 3308, 3011, 2941, 2853, 1628, 1487, 1289, 1126, 789 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.51 (s, 1H, NH), 8.81 (s, 1H, quinazoline H-2), 8.13 (d, J=4.0Hz, 1H, Ph H-2), 8.02 (s, 1H, quinazoline H-5), 7.76 (d, J=8.05Hz, 1H, Ph-H-4), 7.54 (d, J=8.05Hz, 1H, Ph-H-6), 7.50-7.46 (t, J=8.05Hz, 1H, Ph-H-5), 4.05-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 159.0, 154.5, 149.8, 148.2, 138.8, 131.3, 129.7, 127.7, 124.1, 121.8, 109.9, 100.6, 62.7, 61.9, 57.7. Anal. Calcd for C17H16BrN3O3 (390.2): C, 52.32; H, 4.13; N, 10.77. Found: C, 52.21; H, 4.35; N, 10.98.
4-(3-Fluorophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9e). This compound was obtained as pale yellow solid; yield 60.4%, mp 232-234ºC. IR (KBr) n: 3419 , 3013, 2951, 2853, 1628, 1489, 1288, 1134 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.63 (s, 1H, NH), 8.81 (s, 1H, quinazoline H-2), 8.21 (s, 1H, quinazoline H-5), 7.73 (d, J = 10.9 Hz, 1H, Ph-H-4), 7.73 (d, J = 8.0 Hz, 1H, Ph-H-6), 7.57-7.54 (m, 1H, Ph-H-5), 7.20 (t, J = 4.0 Hz, 1H, Ph-H-2), 4.07-4.03 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 161.4, 159.0, 154.6, 149.7, 148.3, 131.0, 130.9, 121.1, 112.5, 112.3, 109.9, 100.7, 62.7, 61.9, 57.8. Anal. Calcd for C17H16FN3O3·HCl (365.8): C, 55.82; H, 4.68; N, 11.49. Found: C, 55.69; H, 4.72; N, 11.35.
4-(3-Fluorophenyl)amino-6,7,8-trimethoxyquinazoline (9f). This compound was obtained as pale yellow solid, yield 36.1%, mp 252-254ºC. IR (KBr) n: 3075, 2934, 2833, 1618, 1454, 1271, 1119 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 9.69 (s, 1H, NH), 8.56 (s, 1H, quinazoline H-2), 7.88 (d, J=12.05 Hz, 1H, Ph-H-4), 7.71 (s, 1H, quinazoline H-5), 7.63 (d, J=8.0 Hz, 1H, Ph-H-6), 7.44-7.43 (m, 1H, Ph-H-5), 6.96 (t, J=4.0 Hz, 1H, Ph-H-2), 4.01-3.93 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 162.7, 160.7, 156.1, 151.4, 146.0, 129.7, 129.6, 117.4, 111.1, 109.6, 108.4, 97.6, 61.6, 60.7, 56.2. Anal. Calcd for C17H16FN3O3 (329.3): C, 62.00; H, 4.90; N, 12.76. Found: C, 61.97; H, 4.85; N, 12.59.
4-(4-Fluorophenyl)amino-6,7,8-trimethoxyquinazoline (9g). This compound was obtained as pale yellow solid; yield 55.4%, mp 184-186ºC. IR (KBr) n: 3356, 3017, 2947, 2857, 1626, 1487, 1283, 1126, 789 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.61 (s, 1H, NH), 8.73 (s, 1H, quinazoline H-2), 8.17 (s, 1H, quinazoline H-5), 7.74-7.71 (m, 2H, Ph-H-3,5), 7.38-7.34 (m, 2H, Ph-H-2,6), 4.05-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 161.7, 159.8, 159.1, 154.5, 149.7, 148.1, 142.1, 133.3, 127.7, 127.6, 116.2, 116.1, 109.7, 100.7, 62.7, 61.8, 57.8. Anal. Calcd for C17H16FN3O3 (329.3): C, 62.00; H, 4.90; N, 12.76. Found: C, 61.85; H, 4.88; N, 12.63.
4-(2-Fluorophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9h). This compound was obtained as pale yellow solid; yield 63.8%, mp 194ºC (dec.). IR (KBr) n: 3364, 2945, 2855, 1630, 1487, 1258, 1126, 1011, 760 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.82 (s, 1H, NH), 8.72 (s, 1H, quinazoline H-2), 8.23 (s, 1H, quinazoline H-5), 7.56-7.36 (m, 4H, Ph-H-3,4,5,6), 4.06-4.03 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 159.3, 157.8, 155.9, 153.9, 149.2, 147.7, 141.7, 129.3, 128.6, 116.3, 116.2, 108.9, 100.2, 62.0, 61.2, 57.1. Anal. calcd for C17H16FN3O3·HCl (329.3): C, 55.82; H, 4.68; N, 11.49. Found: C, 55.66; H, 4.86; N, 11.31.
4-(2-Chloro-4-bromophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9i). This compound was obtained as white solid; yield 66.2%, mp 178ºC (dec.). IR (KBr) n: 3366 , 3015, 2945, 2849, 1626, 1487, 1286, 1126 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 8.71 (s, 1H, quinazoline H-2), 8.04 (s, 1H, quinazoline H-5), 8.00 (d, J = 2.3 Hz, 1H, Ph-H-3), 7.74 (d, J = 8.6 Hz, 1H, Ph-H-5), 7.54 (d, J = 8.6 Hz, 1H, Ph-H-6), 4.03-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 164.8, 159.8, 154.7, 154.4, 153.3, 147.2, 138.8, 137.7, 137.5, 136.6, 126.4, 114.1, 105.1, 104.9, 67.4, 66.7, 62.3. Anal. Calcd for C17H15BrClN3O3·HCl (461.1): C, 44.28; H, 3.50; N, 9.11. Found: C, 43.96; H, 3.76; N, 9.01.
4-(4-Nitrophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9j). This compound was obtained as pale yellow solid; yield 54.3%, mp 212ºC (dec.). IR (KBr) n: 3366, 3011, 2951, 2839, 1628, 1514, 1487, 1340, 1285, 1130 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.21 (s, 1H, NH), 8.82 (s, 1H, quinazoline H-2), 8.36 (d, J = 8.6 Hz, 2H, Ph-3,5-H), 8.15 (d, J = 8.6 Hz, 2H, Ph-2,6-H), 8.06 (s, 1H, quinazoline H-5), 4.06-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 157.8, 153.6, 149.7, 147.4, 143.9, 124.4, 123.2, 110.5, 99.3, 62.0, 61.2, 56.9. Anal. Calcd for C17H16N4O5·HCl (392.8): C, 51.98; H, 4.36; N, 14.26. Found: C, 51.73; H, 4.52; N, 14.12.
4-(3-Nitrophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9k). This compound was obtained as pale yellow solid; yield 34.5%, mp 204-206ºC. IR (KBr) n: 3419, 3015, 2947, 2843, 1632, 1469, 1485, 1346, 1284, 1121 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.84 (s, 1H, NH), 8.86 (s, 1H, quinazoline H-2), 8.72 (s, 1H, Ph-2-H), 8.29 (d, J = 9.15 Hz, 1H, Ph-4-H), 8.26 (s, 1H, quinazoline H-5), 8.18 (d, J = 9.7 Hz, 1H, Ph-6-H), 7.82-7.79 (m, 1H, Ph-5-H), 4.08-4.03 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 159.1, 154.6, 149.8, 148.3, 142.3, 138.6, 131.2, 130.7, 121.3, 119.5, 110.1, 100.8, 62.7, 61.9, 57.9. Anal. Calcd for C17H16N4O5 ·HCl (392.8): C, 51.98; H, 4.36; N, 14.26. Found: C, 51.71; H, 4.55: N, 14.32.
4-(2-Nitrophenyl)amino-6,7,8-trimethoxyquinazoline hydrochloride (9l). This compound was obtained as white solid; yield 32.7%, mp210ºC (dec.). IR (KBr) n: 3019, 2945, 2845, 1624, 1458, 1489, 1356, 1286, 1130, 793 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 12.01 (s, 1H, NH), 8.66 (s, 1H, quinazoline H-2), 8.18 (s, 1H, quinazoline H-5), 8.17 (d, J = 7.45 Hz, 1H, Ph-3-H), 7.90 (t, J = 9.15 Hz, 1H, Ph-4-H), 7.77 (d, J = 7.45 Hz, 1H, Ph-6-H), 7.65 (t, J = 7.75 Hz, 1H, Ph-5-H), 4.06-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.7, 153.9, 149.3, 147.8, 146.3, 144.8, 134.3, 128.9, 128.1, 125.2, 109.3, 99.9, 62.0, 61.2, 57.1. Anal. Calcd for C17H16N4O5·HCl (392.8): C, 51.98; H, 4.36; N, 14.26. Found: C, 52.18; H, 4.57; N, 14.29.
4-(4-Chlorophenyl)amino-6,7,8-trimethoxyquinazoline dihydrochloride (9m). This compound was obtained as pale yellow solid; yield 41.9%, mp 175ºC (dec.). IR (KBr) n: 3420, 3011, 2947, 2853, 1628, 1472, 1286, 1130, 806 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.57 (s, 1H, NH), 8.75 (s, 1H, quinazoline H-2), 8.23 (s, 1H, quinazoline H-5), 7.77 (d, J = 8.6 Hz, 2H, Ph-3,5-H), 7.57 (d, J = 8.6 Hz, 2H, Ph-2,6-H), 4.06-4.02 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.4, 153.7, 149.3, 147.5, 135.8, 128.6, 126.4, 109.9, 100.2, 62.0, 61.2, 57.2. Anal. Calcd for C17H16ClN3O3 ·2HCl (418.7): C, 48.77; H, 4.33; N, 10.04. Found: C, 48.53; H, 4.63; N 9.97.
4-(4-Hydroxyphenyl)amino-6,7,8-triethoxyquinazoline dihydrochloride (9n). This compound was obtained as pale yellow solid; yield 58.0%, mp 153ºC (dec.). IR (KBr) n: 3420, 3227, 3030, 2949, 2855, 1626, 1474, 1273, 1128 cm-1. 1H NMR (DMSO-d6, 500 MHz) d : 11.42 (s, 1H, NH), 9.75 (s, 1H, OH), 8.66 (s, 1H, quinazoline H-2), 8.15 (s, 1H, quinazoline H-5), 7.43 (d, J = 8.6 Hz, 2H, Ph-3,5-H), 6.87 (d, J = 8.6 Hz, 2H, Ph-2,6-H), 4.03-4.00 (t, 9H, 3OCH3). 13C NMR (DMSO-d6, 125 MHz) d : 158.3, 156.1, 153.7, 149.1, 147.3,141.6, 127.6, 126.3, 115.2, 109.1, 100.2, 61.9, 61.2, 57.1. Anal. Calcd for C17H17N3O4 ·2HCl (400.3): C, 51.01; H, 4.78; N, 10.50. Found: C, 51.24; H, 5.04; N, 10.52.
3. RESULTS AND DISCUSSION
3.1 The selection of nitration route
In the first imagining, we thought that 2-amino-3,4,5-trimethoxybenzoic acid (6) was
synthesized by etherification, nitration, and then hydrolyzation, deoxidization starting
from gallic acid (1) as raw material. But, 3,4,5-trimethoxynitrobenzene was obtained
instead of 2-nitro-3,4,5- trimethoxybenzoic acid (5) in nitration step, conforming the
results by IR, 1H NMR, 13C NMR and elemental analysis. The mechanism
of decarboxylation may be seen in Scheme 2. So, 2-nitro-3,4,5- trimethoxybenzoic acid (5)
was synthesized by etherification, esterification, and then nitration, hydrolyzation
starting from gallic acid (1) as raw material. Decarboxylation can be easily avoided using
this method.
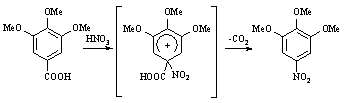
Scheme 2
3.2 The selection of nitration reagent
It can be found from Table 1 that different nitration
reagents and reaction solvents affected the yield of methyl
2-nitro-3,4,5-trimethoxybenzoate (4). It is very clear that there were better yield
with fuming nitric acid than that yield with concentrated nitric acid as nitration reagent when the
same solvent is acetic acid. When the solvent was changed from acetic acid, concentrated
sulphuric acid to without solvent, the yield of nitration was still lower than that the
yield with fuming nitric acid as nitration reagent.
Table 1 The effect on yield in different nitration condition
Nitration Reagent |
Solvent |
Yield/% |
mp/ ºC |
con. HNO3 |
CH3COOH |
14.6 |
57-60 |
con. HNO3 |
con. H2SO4 |
5.8 |
59-62 |
con. HNO3 |
――a |
16.3 |
58-62 |
fuming HNO3 |
CH3COOH |
25.4 |
62-63 |
Note: a no solvent.
3.3 Selection of deoxidization route
If methyl 2-nitro-3,4,5-trimethoxybenzoate (4) was deacidized straightforward, methyl 2-amino-3,4,5-trimethoxybenzoate expected could not be obtained, instead its salt stannic chloride (10) formed (Scheme 3).
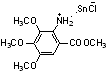
10
Scheme 3
Compound 10: This compound was obtained as white cystal; yield 74.3%, mp 180
ºC (dec.). 1H NMR (DMSO-d6, 500 MHz) d: 7.17 (s, 1H, PhH), 4.38 (s, 2H, NH2), 3.86 (s, 3H, COOCH3), 3.83-3.77 (s, 9H, 3OCH3); 13C NMR (DMSO-d6, 125 MHz) d : 167.0, 147.2, 146.5, 142.9, 134.0, 109.0, 108.9, 61.3, 61.1, 56.7, 52.5. IR (KBr) n : 3200-3200 (nNH2+nPh-H+nCH3), 3099.6 (nPh-H), 2947.2 (nasCH3), 2846.9 (nsCH3), 1703.1 (nC=O), 1587.4-1454.3 (Ph skeleton vibration), 1249.9 (nasPh-O-C), 1132.2 (nsPh-O-C), 775.4 (dPh-H) cm-1. Anal. calcd for C11H15ClNO5Sn: C 33.41, H 3.82, N 3.54; found C 33.34, H 3.58, N 3.52.Finally, 2-amino-3,4,5-trimethoxybenzoic acid (6) was synthesized by etherification, esterification, nitration, hydrolyzation, deoxidization, starting from gallic acid (1) as raw material. The synthesis of 6 was described in the text.
4. CONCLUSION
Fourteen novel 6,7,8-trimethoxy N-substituted-4-aminequinazoline compounds were
synthesized starting from gallic acid that is natural material. All of the structures of
title products were characterized by IR, 1H NMR, 13C NMR and
elemental analysis. Meanwhile, the preliminary research for the effects of reaction
conditionson intermediates synthesis was performed.
REFERENCES
[1] Szczepankiewicz W, Suwinski J, Bujok R. Tetrahedron, 2000, 56 (47): 9343.
[2] Tobe M, Isobe Y, Tomizawa H, et al. Bioorg. Med. Chem,. 2003, 11 (4): 609.
[3] Liu G, Yang S, Song B-A, et al. Molecules, 2006, 11: 272.
[4] Rosowsky A, Papoulis A T, Forsch R A, et al. J. Med. Chem., 1999, 42 (6): 1007.
[5] Jackman A L, Kimbell R, Brown M, et al. Adv Exp Med Biol., 1994, 370: 185.
[6] Gangjee A, Zaveri N, Kothare M, et al. J. Med. Chem., 1995, 38 (18): 3660.
[7] Griffin R J, Srinivasan S, Bowman K, et al. J. Med. Chem., 1998, 41 (26): 5247.
[8] Smaill J B, Rewcastle G W, Loo J A, et al. J. Med. Chem., 2000, 43 (7): 1380.
[9] Wissner A, Berger D M, Boschelli D H, et al. J. Med. Chem., 2000, 43 (17): 3244.
[10] Bridges A J, Zhou H, Cody D R, et al. J. Med. Chem., 1996, 39 (1): 267.
[11] Discafani C M, Carroll M L, Floyd M B, et al. Biochem. Pharmacol., 1999, 57 (8): 917.
[12] Rewcastle G W, Palmer B D, Bridges A J, et al. J. Med. Chem., 1996, 39 (4): 918.
[13] Mauthner F, Clarker H-T. Organic Synthesis Collection, 1st ed., Vol. 1;
Gilman, H., Ed.; Wiley, New York, 1941, pp 537.
[14] Kudryashova N I, Davidenko L R, Khromov-Borisov N V. Zh. Obshch. Khim. 1959, 29:
1885.
新型6,7,8-三甲氧基-N-取代-4-胺基喹唑啉类化合物的合成和表征
刘刚, 孙琳, 刘春萍, 纪春暖
(鲁东大学化学与材料科学学院, 山东烟台 264025)
摘要 以天然产物没食子酸为原料,6,7,8-三甲氧基-4-氯喹唑啉和芳香胺在异丙醇回流条件下反应生成新型6,7,8-三甲氧基-N-取代-4-胺基喹唑啉类化合物。目标化合物的结构经元素分析、红外光谱、核磁共振氢谱和核磁共振碳谱进行了表征。同时,对于中间体的合成条件进行了初步探讨。
关键词 喹唑啉,没食子酸,合成