http://www.chemistrymag.org/cji/2008/104019pe.htm |
Apr. 1,
2008 Vol.10 No.4 P.19 Copyright |
(1 Yunnan Academy of Tobacco Science, Kunming 650106; 2Life science school,Yunnan University, Kunming 650091, China)
Abstrasct A new chromogenic reagent,
2-(2-quinolylazo)-5-dimethylaminoaniline (QADMAA) was synthesized. A sensitive, selective
and rapid method for the determination of palladium based on the rapid reaction of
palladium(II) with QADMAA was developed. In the presence of 0.5-2.5 mol/L of hydrochloric
acid solution and cetyl trimethylammonium bromide (CTMAB) medium, QADMAA reacts with
palladium to form a violet complex of a molar ratio 1:2 (palladium to QADMAA). The molar
absorptivity of the chelate is 1.35×105 L.mol-1.cm-1 at
600 nm. Beer’s law is obeyed in the range of 0.01~
0.6 m g/ml. The relative standard deviation for eleven replicate sample of 0.2 m g/ml
level is 1.02 %. This method had been applied to the determination of palladium with good
results.
Keywords 2-(2-quinolylazo)-5-dimethylaminoaniline; palladium; spectrophotometry
1. INTRODUCTION
Palladium is an important element. It is important for industry and biological systems
as well [1-2]. Many sensitive instruments, such as spectrofluorimetry, X-ray
fluorescence spectrometry, neutron activation analysis, atomic absorption spectrometry,
chemiluminescence, and the like have widely been applied to the determination of
palladium. But the spectrophotometric method still has the advantages of being simple and
not requiring expensive or complicated test equipment. For this reason, a wide variety of
spectrophotometric methods for the determination of palladium have been developed [3-6].
In our previous work, some 2-quinolylazo-phenol reagents were reported
for the determination of metal ions [7-9]. This kind reagent has a higher
sensitivity than pyridylazo reagents because of its larger conjugated system. However, the
2-quinolylazo-phenol reagent has also a disadvantage of its poor selectivity because both
the oxygen atoms and nitrogen atoms donate to the metal ions. To select a more sensitive
and selective reagent, we synthesized 2-(2-quinolinylazo)-5-dimethylaminoaniline (QADMAA)
and thoroughly studied the color reaction of QADMAA with palladium. This reagent has a
higher selectivity than 2-quinolylazo-phenol reagents because of it only donating nitrogen
atoms to metal ions. Based on the color reaction of QADMAA with palladium, a highly
sensitive, selective and rapid method for the determination of palladium was
developed.
2. EXPERIMENTAL
2.1 Apparatus
A UV-160 A spectrophotometer (Shimidzu Corporation, Tokyo, Japanese) equipped with 1
cm cells was used for all absorbance measurements. The pH values were determined with a
Beckman Φ-200 pH meter (Beckman Instruments,
Fullerton, CA, USA).
2.2 Synthesis of QADMAA
2-Aminoquinoline (6.9 g) was dissolved in 500 mL anhydrous ethanol. To which, sodamide
(2.0 g) was added and the mixture was refluxed in boiling water bath for 5 h, followed by
the addition of isoamyl nitrite (7.4 mL). The solution was refluxed for 30 min with
boiling water bath. The solution was cooled and placed over night under 0℃. The diazo salt was obtained by filtering this solution with an
isolation yield of 95%. The diazo salt was dissolved in 200 mL anhydrous ethanol, followed
by the addition of m-dimethylaminoaniline (5.7g; 0.042 mol). The carbon dioxide was
ventilated into the solution with stirring until the pH reaches to about 8.0. The solution
stood for two days, and evaporated to dryness. The residue was re-crystallized with 30%
ethanol. QADMAA was obtained with 36% yield. The structure of QADMAA was verified by
elemental analysis, IR, 1HNMR, and MS.
2.3 Reagents
All of the solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP
Reagent Water System (Millipore Corporation, USA). A 5´ 10-4 mol/L of
QADMAA solution was prepared by dissolving QADMAA with 95% of ethanol. A stock standard
solution of palladium (1.0 mg/mL) was obtained from Chinese Standard Center, and a work
solution of 2.0 m g/mL was prepared by diluting this solution. A 5 mol/L of hydrochloric
acid was used. Cetyl trimethylammonium bromide (CTMAB) solution (1.0 % (w/v)) was prepared
by dissolving CTMAB with 20% ethanol. All chemical used were of analytical grade unless
otherwise stated.
2.4 General procedure
To a standard or sample solution containing no more than 15 m g of Pd(II) in a 25 mL of
calibrated flask, 5 mL of 5 mol/L hydrochloric acid, 4.0 mL of 5×10-4 mol/L
QADMAA solution and 2.0 mL of 1.0 % CTMAB solution were added. The mixture was diluted to
volume of 25 mL and mixed well. After 10 min, the absorbance was measured in a 1 cm cell
at 600 nm against a reagent blank prepared in a similar way without palladium.
3. RESULTS AND DISCUSSION
3.1 Absorption Spectra
The absorption spectra of QADMAA and its Pd(II) complex are shown in Fig.1. The
absorption peaks of QADMAA and its complex are located at 440 nm and 600 nm.
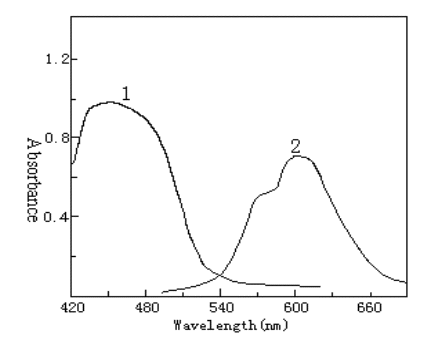
Fig.1 Absorption spectra of QADMAA and its Pd(II) complex
1 QADMAA-CTMAB blank against water; 2 QADMAA-Pd(II)-CTMAB complex against reagent blank
3.2 Effect of Acidity
Results showed that the optimal condition for the reaction of Pd(II) with QADMAA is in the
acid medium. Therefore, the effect of hydrochloric acid, sulfuric acid, perchioric acid,
phosphoric acid and the like, on the color reaction of Pd(II) with QADMAA was studied.
Experiment shows that hydrochloric acid has the best effect, and the concentration of
hydrochloric acid within 0.5-2.5 mol/L was found to give a maximum and constant
absorbance. So 5 ml of 5 mol/L of hydrochloric acid was recommended.
3.3 Effect of surfactants
The effect of surfactants on Pd(II)-QADMAA chromogenic system was studied. In the presence
of cationic surfactants or nonionic surfactants medium, the absorption of the chromogenic
system increases markedly. Experiments show that CTMAB is the best additive. The use 1~ 4
mL of 1.0 % CTMAB solution give a constant and maximum absorbance. Accordingly, the use of
2 mL was recommended.
3.4 Effect of QADMAA concentration
For up to 15 m g of Pd(II), the use of about 3.5~ 5 mL of 5×10-4 mol/L of
QADMAA solution has been found to be sufficient for a complete reaction. Accordingly, 4 ml
of QADMAA solution was added in all further measurement.
3.5 Stability of the chromogenic system
After mixing the components, the absorbance reaches its maximum within 8 min at room
temperature and remains stable for 6 h in aqueous solution. The chelates are stable at
least 20 h.
3.6 Calibration Curve and Sensitivity
The calibration curve show that Beer's law is obeyed in the concentration range of
0.01~ 0.6 m g Pd(II) per ml, The linear regression equation obtained was: A = 1.294 C (m
g/mL) + 0.0158, (r=0.9996). The molar absorptivity was calculated to be 1.35×105 l.mol-1.cm-1
at 600 nm. The relative standard deviation at a concentration level of 0.2 m g Pd(II) per
mL (11 repeat determination) was 1.02 %.
3.7 The method precision and recovery
The intra-day precision and inter-day precision were calculated from
data obtained during a 7-day validation. The precision of the assay was determined by
repeatability (intraday) and intermediate precision (inter-day). To assess intraday
variation (repeatability), calibration curve was prepared seven times on the same day.
Intermediate precision was assessed by comparing the assays on different days (7 days, n =
7 at each concentration). The results showed that the relative standard derivation of
overall intra-day variations were less than 2.5%, and the relative standard derivation of
inter-day variations were less than 2.8%. This method is high precision.
The recovery test of the proposed method was prepared by adding a known
amount of standard at three different levels (1.0 m g/mL, 2.0 m g/mL, 5.0 m g/mL) to the
pre-analysed sample. The results shown that the recoveries (n=7) were ranged from 94% -
105%. This method is high recovery.
3.8 Composition of the complex
The composition of the complex was determined by molar ratio method (Figure 2) and
continuous variation (Figure 3). Both showed that the molar ratio of Pd(II) to QADMAA is
1:2.
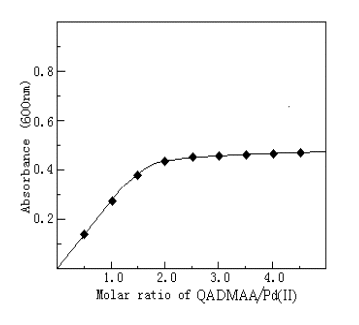
Fig.2 Composition of QADMAA-Pd(II)
complex by molar ratio method
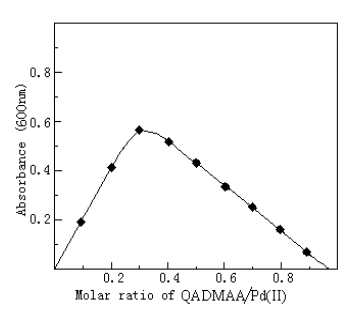
Fig. 3 Composition of QADMAA-Pd(II) complex by continuous variation method
3.9 Interference
The selectivity of the proposed method was investigated by the determination 5 m
g/25ml of Pd(II) in the presence of various ions within a relative error of ± 5% are
given in Table-1. The results showed that most foreign ions do not interfere
with the determination. This method is high selectivity.
Table 1 Tolerance limits for the determination of 5 m g of Pd(II) with QADEAA (relative error ± 5% )
Ion added |
Tolerate (mg) |
NO3-, K+, borate, Na, Cl-, Mg2+, SO42-, ClO4- |
200 |
Li+, Al3+, PO43-, NO2-, ClO3- |
20 |
Ca2+, Sr2+, IO3-, BrO3-, B(III) |
10 |
Mn2+, Ce(IV) , Fe3+, Mo(VI), V(V) |
5 |
Ti(IV), Bi(III), Cr(VI), Ba2+ W(WI), U(IV), [Co2+]* |
1 |
Cd2+, Pd2+, Cr3+, La3+, Zn2+, {Cu2+]*, Zr(IV), [Ni2+]* |
0.5 |
Bi(III), Pb2+, Hg 2+, Sb3+, Th(IV), Sn(IV) |
0.1 |
Se(IV), Te(IV), Au3+, Cu2+, Ag+ |
0.05 |
Ni2+, Co2+ |
0.01 |
| * masked with 2 mL of 10% citric acid | |
4. APPLICATION
The proposed method has been successfully applied to the determination of palladium in
water and katalyst.
For water sample, taking an appropriate volume (planting effluents 20
ml, river water 500 ml) of water sample in a 500 mL flask. The samples were concentrated
to about 10 mL by heating on a hot plate. 5 mL concentrated nitric acid and 2 mL of 30%
hydrogen peroxide was added in this solution. The mixture was heated on a hot plate and
evaporated to near dryness. The residue was dissolved with 5 mL of 5% hydrochloric acid
and transferred into a calibrated flask, and 2 mL of 10% citric acid was added to mask the
nickel and cobalt. The solution was neutralized with sodium hydroxide, and analyzed by
general procedure. The recovery of palladium was determined by adding 1.0 m g of palladium
to water samples. A standard method using atomic absorption spectrometry was also used as
reference method. The results are shown in Table 2.
For katalyst, 0.1 g of samples was weighted accurately into the Teflon
high-pressure microwave acid-digestion bomb (Fei Yue, Analytical Instrument Factory,
Shanghai, China). 3.0 ml of concentrated nitric acid, 2 mL of hydrochloric acid and 5.0 ml
of 30% hydrogen peroxide were added. The bombs were sealed tightly and then positioned in
the carousel of the microwave oven (Model WL 5001, 1000 W, Fei Yue Analytical Instrument
Factory, Shanghai, China). The system was operated at full power for 10 min. The digest
was evaporated to near dryness. The residue was dissolved with 10 ml of 10% of
hydrochloric acid, then transferred into a 50 ml of calibrated flask and diluted to volume
with 10% of hydrochloric acid. The palladium content was analyzed by general procedure.
The recovery of palladium by added 1.0 m g of palladium in sample was carried out, and a
standard method using atomic absorption spectrometry was also used as reference method.
The results are shown in Table 2.
Table 2 Determination of palladium in the water sample
Samples |
AAS method |
Found |
RSD% |
Recovery (%) |
River water |
0.0182 (m g/mL) |
0.0156 (m g/mL) |
2.3 |
103 |
Planting effluents |
0.248 (m g/mL) |
0.237 (m g/mL) |
2.1 |
96 |
| katalyst | 0.532 (%) |
0.536 (%) |
1.2 |
101 |
REFERENCE
[1]W. Bauer, W. Beck, and W. Ponikwar. Zeitschrift fur Naturforschung. 2000,
55:946-952
[2]G. Patrick, and P. Michel. Platinum Metals review. 2003, 47: 60-72
[3]B. Maria, and P. Katarzyna. Chem.Anal. 2003, 48:87-95
[4]L.S. Sarma, J.R. Kumar, K.J. Reddy, et al.. Anal.Sci. 2002,18, 1257-1261
[5]D.L. Ma, F.L. Cui, D.S. Xia, and Y.L. Wang, Anal. Lett. 2002, 35: 413-421
[6]D. Gholivand, and B. Mohammad, Talanta. 2000, 52: 1055-1060
[7]Q.F. Hu, G.Y. Yang, J.Y. Yin, J.Environ.Monit. 2002, 4:956-959
[8]G.Y. Yang, Q.F. Hu, G.Y. Yang, et al.. Anal. Sci. 2003, 19: 299-302
[9]G.Y. Yang, Z.J. Huang, Q.F. Hu, et al.. Talanta. 2002, 58: 511-515
胡群1,胡琳2,王建1,魏玉玲1,陈永宽1
(1云南烟草科学研究院 昆明650106;2云南大学生命科学学院,昆明650091)
摘要 研究了新试剂,2-(2-喹啉偶氮)-5-二甲基胺苯胺(QADMAA)与钯的显色反应。基于钯(II)与QADMAA的快速反应,确定了一种具有选择性和快速测定钯的检测方法。在0.5-2.5 mol/L 的盐酸溶液和溴化十六烷基三甲胺(CTMAB)存在下,QADMAA与钯反应生成摩尔比(钯/QADMAA)为1:2的紫色的络合物。在吸收波长600 nm下,摩尔吸光系数 e= 1.35×105L.mol-1.cm-1, 比尔法测定钯的浓度范围为0.01~ 0.6 m g/ml。通过11个钯含量为0.2 m g/ml的复杂样品的测定,该方法的相对标准偏差为1.02 %。此方法用于测定样品中的钯,结果令人满意。
关键词 2-(2-喹啉偶氮)-5-二甲基胺苯胺;钯;光度法