http://www.chemistrymag.org/cji/2008/109047pe.htm |
Sep.
1, 2008 Vol.10 No.9 P.47 Copyright |
Spectroscopic studies on the interaction of 3,3'-diselenadibenzoic acid with human serum albumin
Sun
Shaofaa,b , Hou Hannab, Wu Minghua, Ding Xinliangb, Chen Xusheng b
(a Department of Chemistry and Life Sciences, Xianning College, Xianning
437005;b College of Chemistry and Molecular Sciences, Wuhan University, Wuhan
430072, China)
Supported by the Natural Science Foundation of Hubei Province(No.2006ABA333), and Science Foundation of Hubei Provincial Educational Department (D200528003)
Abstract The interaction between
3,3'-diselenadibenzoic and Human serum albumin (HSA) was investigated by means of
fluorescence and absorption spectroscopy. The quenching mechanism of fluorescence of HSA
by 3,3'-diselenadibenzoic acid was discussed. It is proved that the fluorescence quenching
of HSA by 3,3'-diselenadibenzoic acid is a result of the formation of
HSA-3,3'-diselenadibenzoic acid complex. The binding sites number (n), apparent
binding constant (K) and corresponding thermodynamic parameters, such as ![]() ,
, ![]() , and
, and![]() etc., were calculated. Results indicate that the electrostatic
interactions forces played major role in the reactions.
etc., were calculated. Results indicate that the electrostatic
interactions forces played major role in the reactions.
Keywords Human serum albumin (HSA), 3, 3'-diselenadibenzoic acid, Fluorescence
quenching, Thermodynamic parameters
1. INTRODUCTION
Serum albumin is the most abundant proteins in the circulatory system of a wide
variety of organisms, being the major macromolecule contributing to the osmotic blood
pressure;1 they can play a dominant role in drug disposition and efficacy.2 Many drugs and
other bioactivity small molecules bind reversibly to albumin and other serum components
that then function as carriers. Serum albumin often increases the apparent solubility of
hydrophobic drugs in plasma and modulates their delivery to cells in-vivo and in-vitro.
Consequently, it is important to know the affinity of a drug for serum albumin even if it
is not the only factor predictive of serum concentrations of the free drug.
Fluorescence spectroscopy is a powerful tool for the study of the
reactivity of chemical and biological systems. Quenching measurement of albumin
fluorescence is an important method to study interactions of several substances with this
protein.3
Selenium, as an essential trace element, is of fundamental importance
to human health. It appears to be a key nutrient in counteracting the development of
virulence and inhibiting HIV progression to AIDS.4 Therefore many organoselenium compounds
have been synthesized and their bioactivities have been studied.5 Also our previous
studies showed that the anti-microbial activity of organoselenium compounds is many times
higher than that of sulfur and oxygen analogs having isosteric elements.6
In this work, we studied in vitro interaction of 3,3'-diselenadibenzoic
acid (Fig.1) with HSA using the quenching of intrinsic fluorescence of tryptophan
residues. The aim of our work was to estimate the interaction mechanism, and to
investigate the thermodynamics of their interaction.
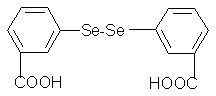
Figure 1. Molecular structure of 3,
3'-diselenadibenzoic acid
2. EXPERIMENTAL
2.1 Materials
3,3'-diselenadibenzoic acid was synthesized and characterized by our group. Human
serum albumin, being electrophoresis grade reagents, were obtained from Sigma. The buffer
Tris had a purity of no less than 99.5% and NaCl, HCl, etc. were all of analytical purity.
The samples were dissolved in Tris-HCl buffer solution (0.05 mol·dm-1 Tris, 0.10
mol·dm-1 NaCl, pH = 7.4). The solutions of albumin were prepared 30 minutes before
experiment. All solutions were used with doubly distilled water.
2.2 Apparatus and Measurements
Fluorescence spectra were recorded on F-2500 Spectrofluorimeter (Hitachi, Japan) with 1×1×4cm
cell. Addtionally the circulating water bath was used to maintain temperature, the
fluorescence measurements were performed at different temperatures (298, 302, 306 and
310K). Excitation wavelength was 285 nm. The excitation and emission slit widths were set
at 2.5 nm. Appropriate blanks corresponding to the buffer were subtracted to correct
background of fluorescence.
TU-1901 spectrophotometer ( PuXi Ltd of Beijing, China ) was used to
measure absorption spectra. The absorption spectra of HSA, 3, 3'-diselenadibenzoic acid
and their mixture were performed at room temperature.
The mass of the sample was accurately weighted using a microbalance
(Sartorius, ME215S) with a resolution of 0.1 mg.
3. RESULTS AND DISCUSSION
3.1 Fluorescence spectra and quenching mechanism
The concentrations of HSA were stabilized at 1.0×10-5 mol·L-1, and the content
of 3, 3'-diselenadibenzoic acid varied from 0 to 1.2×10-5 mol·L-1 at the step of
0.2×10-5 mol·L-1. The effects of 3, 3’-diselenadibenzoic
acid on HSA fluorescence intensity are shown in Fig. 2.
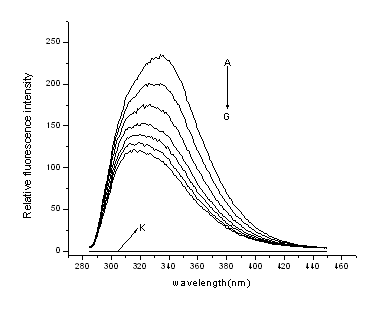
Figure 2. Emission spectra of HSA in the
presence of various concentrations of 3, 3'-diselenadibenzoic acid
T=298K, lex=285nm; c
(HSA)= 1.0×10-5 mol·L-1; c (3,3'-diselenadibenzoic acid) / (10-5 mol·L-1),
A-G: 0; 0.2; 0.4; 0.6; 0.8; 1.0; 1.2; curve K shows the emission spectrum of
3,3'-diselenadibenzoic acid only( c = 1.0×10-5 mol·L-1).
From Fig. 2, it is
apparent that the fluorescence intensity of HSA decreased regularly with the increasing of
3,3'-diselenadibenzoic acid concentration.
Quenching can occur by different mechanisms, which usually classified
as dynamic quenching and static quenching. Dynamic and static quenching can be
distinguished by their differing dependence on temperature and viscosity. Higher
temperatures result in faster diffusion and hence larger amounts of collisional quenching.
Higher temperatures will typically result in the dissociation of weekly bound complexes,
and hence smaller amounts of static quenching. 7
In order to confirm the quenching mechanism, the fluorescence quenching
data disposed by us was assumed to be dynamic quenching. For dynamic quenching, the
decrease in intensity is described by the well-known Stern-Volmer equation3, 8:
![]() (1)
(1)
Where, F0 and F are the steady-state fluorescence
intensities in the absence and in the presence of quencher (3,3'-diselenadibenzoic acid), ![]() is the Stern-Volmer quenching constant, Kq is
the bimolecular quenching constant,
is the Stern-Volmer quenching constant, Kq is
the bimolecular quenching constant, ![]() is the unquenched lifetime,
and
is the unquenched lifetime,
and ![]() is the concentration of quencher.
is the concentration of quencher.
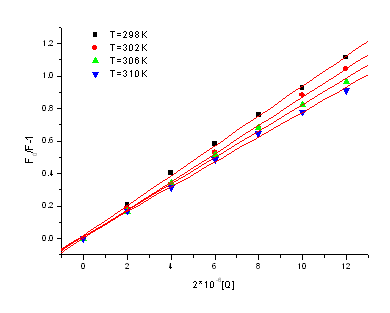
Figure 3. The line relation of (F0/F-1)
and [Q] at different temperatures
Figure 3 displays the
Stern-Volmer plots of the quenching of HSA tryptophan residues fluorescence by
3,3'-diselenadibenzoic acid at different temperatures. From the corresponding Stern-Volmer
quenching constant and the linear relation at different temperatures, we conclude the
probable quenching mechanism of fluorescence of HSA by 3,3'-diselenadibenzoic acid are not
initiated by dynamic collision but by formation of complex.
For static quenching, the decrease in intensity is described by the
Lineweaver-Burk equation: 9
![]() (2)
(2)
In the present case, KLB is the formation constant. The
corresponding Lineweaver-Burk formation constant and the linear relation at different
temperatures are shown in Table 1. It shows that the binding constant between
3,3'-diselenadibenzoic acid and HSA is great and the effect of temperature is small. Thus,
3,3'-diselenadibenzoic acid can be stored and carried by protein in the body.
Table 1 Lineweaver-Burk quenching constant and the linear relation of KLB and T at different temperatures
pH |
T/K |
Linear regress equations |
aR |
10-4KLB/L·mol-1 |
7.40 |
298 |
(F0 - F)-1-1=0.0044+3.6810-4[Q]-1 |
0.9999 |
3.069 |
302 |
(F0 - F)-1-1=0.0049+4.3910-4[Q]-1 |
0.9970 |
2.884 |
|
306 |
(F0 - F)-1-1=0.0041+4.8910-4[Q]-1 |
0.9997 |
2.765 |
|
310 |
(F0 - F)-1-1=0.0049+4.9610-9[Q]-1 |
0.9992 |
2.606 |
aR is the linear correlated coefficients
3.2 Analysis of binding
equilibria
When small molecules bind independently to a set of equivalent sites on a macromolecule,
the equilibrium between free and bound molecules is given by the equation:8
![]() (3)
(3)
Where, in the present case, K is the binding constant to a site,
and n is the number of binding sites per serum albumin. According to the equation
(3), the fit of the fluorescence date for the system of 3,3’- diselenadibenzoic acid and HSA show that n is almost a
constant at four different temperature, we can conclude that 3,3’-diselenadibenzoic acid molecule formed 1:1 complex with HSA
molecule. Figure 4 is the fit between log((F0-F)/F) and log[Q] at
four different temperatures. From figure 4, it can be seen that the value of KLB
and n decreased slightly with the temperatures rising, which may indicates that
there is molecular binding with HSA and forming an unstable compound. The unstable
compound would be partly decomposed when the temperature rising, therefore, the values of K
and n decreased slightly with the temperatures rising.
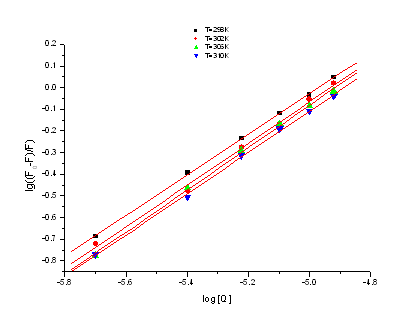
Figure 4. The fit between log((F0-F)/F)
versus log[Q] at four different temperatures
3.3 Thermodynamic parameters
In order to elucidate the interaction of 3,3'-diselenadibenzoic acid with HSA, the
thermodynamic parameters were calculated from the Van't Hoff plots. If the enthalpy change
(![]() ) does not vary significantly over the
temperature range studied, then its value and that of entropy change (
) does not vary significantly over the
temperature range studied, then its value and that of entropy change (![]() ) can be determined from the Van't Hoff equation:
) can be determined from the Van't Hoff equation:
![]() (4)
(4)
Where K is the binding constant (KLB) at the
corresponding temperature and R is the gas constant. The temperatures used were
298, 302, 306 and 310 K. The enthalpy change (![]() ) is calculated from the slope of the Van’t Hoff relationship. The free energy change (
) is calculated from the slope of the Van’t Hoff relationship. The free energy change (![]() ) is estimated from the following relationship:
) is estimated from the following relationship:
![]() (5)
(5)
Fig.5, by fitting the data of Table 1, shows that assumption of near
constant ![]() is justified. The values of
is justified. The values of ![]() and
and ![]() obtained for the binding sites from the slopes and ordinates at the origin of
the fitted lines are shown inTable 2. From Table2, it can be seen that the negative sign
for free energy (
obtained for the binding sites from the slopes and ordinates at the origin of
the fitted lines are shown inTable 2. From Table2, it can be seen that the negative sign
for free energy (![]() ) means that the binding process is spontaneous.
The negative enthalpy (
) means that the binding process is spontaneous.
The negative enthalpy (![]() ) and positive
entropy (
) and positive
entropy (![]() ) values of the interaction of 3,3’-diselenadibenzoic acid and BSA indicate that the electrostatic
interactions forces played major role in the reactions.9
) values of the interaction of 3,3’-diselenadibenzoic acid and BSA indicate that the electrostatic
interactions forces played major role in the reactions.9
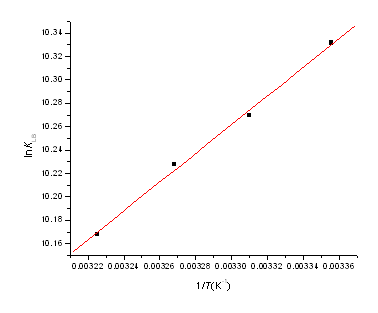
Figure 5. Van't Hoff plot, pH 7.40; c(HSA)=
1.0×10-5 mol·L-1
Table 2. Binding constant (KLB )and relative thermodynamic parameters
pH |
T |
10-4KLB /L·mol-1 |
DH / kJ·mol-1 |
DG / kJ.mol-1 |
DS / J·mol-1·K-1 |
| 7.4 |
298 302 306 310 |
3.069 2.884 2.765 2.606 |
- 10.21 |
-25.59 -25.80 -26.01 -26.22 |
51.63 |
3.4 Energy transfer from HSA to
3, 3'-diselenadibenzoic acid
The Förster theory of molecular resonance energy transfer10 point out: in addition to
radiation and reabsorption, a transfer of energy could also take place through direct
electrodynamic interaction between the primarily excited molecule and its neighbors.
According to this theory, the distance r of binding between 3,3'-diselenadibenzoic
acid and serum albumins could be calculated by the equation.11
![]() (6)
(6)
Where E is the efficiency of transfer between the donor and the
acceptor, R0 is the critical distance when the efficiency of transfer is 50%.
![]() (7)
(7)
In equation (7), K2 is the space factor of orientation; N
is the refracted index of medium; f is the fluorescence quantum yield of the donor; J is the
effect of the spectral overlap between the emission spectrum (b) of the donor and the
absorption spectrum (a) of the acceptor (Fig. 6), which could be calculated by the
equation:
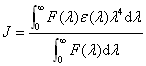 (8)
(8)
Where, F(l) is the corrected fluorescence intensity of the donor in the
wavelength rangel
tol+Dl; e(l) is the extinction
coefficient of the acceptor at l. The efficiency of transfer (E) could be obtained by the
equation:
![]() (9)
(9)
In the present case, K2= 2/3, N =1.36, f= 0.15, 12according to the
equation (6) - (9), we could calculate that R0 = 3.64 nm; E =
0.47 and r = 3.71 nm. The distance r﹤8
nm,13, 14 indicate that the energy transfer from BSA to 3,3'-diselenadibenzoic acid occurs
with high probability.
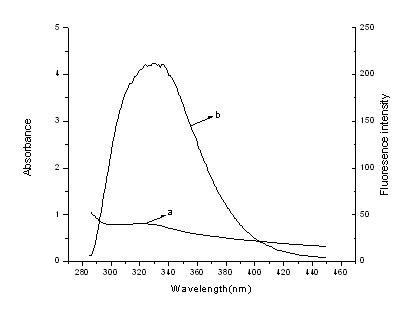
Figure 6. Spectral overlap of the
fluorescence of HSA(b) and the absorption of 3,3'-diselenadibenzoic acid(a)(CHSA=C(3,3'-diselenadibenzoic
acid)=1.0×10-5 mol·L-1))
4. CONCLUSIONS
The interaction of 3,3'-diselenadibenzoic acid with HSA was studied by Spectroscopic
methods including fluorescence spectroscopy and UV-visible absorption spectroscopy. The
experimental results also indicate that the probable quenching mechanism of fluorescence
of HSA by 3,3'-diselenadibenzoic acid is a constant quenching procedure. From the number
binding sites we can conclude that 3,3'-diselenadibenzoic acid molecule formed 1:1 complex
with HSA molecule. The enthalpy change (![]() )
and the entropy change (
)
and the entropy change (![]() ) were also calculated. From the two
parameters we know that the electrostatic interactions forces played major role in the
reaction. The main purpose of this research is to study the binding properties between HSA
and 3,3'-diselenadibenzoic acid for the great importance in pharmacy, pharmacology and
biochemistry. As a kind of pharmaceutical that is not used orally, this experiment can
supply some important information to clinically research.
) were also calculated. From the two
parameters we know that the electrostatic interactions forces played major role in the
reaction. The main purpose of this research is to study the binding properties between HSA
and 3,3'-diselenadibenzoic acid for the great importance in pharmacy, pharmacology and
biochemistry. As a kind of pharmaceutical that is not used orally, this experiment can
supply some important information to clinically research.
REFERENCES
[1] D C Carter, J X Ho. Adv. ProteinChem, 1994, 45:153-203.
[2] R E Olson, D D Christ. Ann. Rep. Med. Chem. 1996,31:327-337.
[3] J R Lakowicz. Principles of Fluorescence Spectroscopy, second ed. (New York: Kluwer
press, 1999, 237-265.
[4] M P Rayman. The Lancet, 2000, 356:233-241.
[5] H B Xu, K X Huang. Chemistry and Biochemistry of Selenium and its Application in Life
Sciences, Huazhong: Huazhong University of Science and Technology Press, 1994 .
[6] X Li. Microcalorimetric study of interaction between selenium compounds and cell or
mitochondria, Ph.D. Thesis, Wuhan University,2001.
[7] R Joseph, J R Lakowicz. Principles of Fluorescence Spectroscopy (2nd edition), New
York: Plenum press , 1999.
[8] Z F Xi, C L Zhang. Talanta , 1998,47:1223-1229.
[9] D P Ross , S Subramanian. Biochemistry , 1981, 20:3096-3102.
[10] T F?rster. Ann. Phys. 1948, 2:55-75.
[11] Y V Il'ichev , J L Perry, J D Simon. J. Phys. Chem. B, 2002,106:452–459.
[12] L Cyril, J K Earl, W M Sperry. Biochemists Handbook, London: Epon Led, , 1961.
[13] B Valeur, J C Brochon. New Trends in Fluorescence Spectroscopy (6th edition),
Berlin :Springer, 1999.
[14] Z F Xi, C L Zhang. Talanta, 1998, 47:223-1229.
孙绍发a,b,吴鸣虎a,候汉娜b
(a咸宁学院化学与生命科学系, 湖北咸宁, 437005; b武汉大学化学与分子科学学院, 湖北武汉, 430072)
摘要 光谱法研究3, 3’-二硒苯二甲酸与人清白蛋白的相互作用,实验发现3, 3’-二硒苯二甲酸能强烈猝灭牛血清白蛋白的荧光,其荧光猝灭机理为静态猝灭。分析并处理实验数据,计算了二者相互作用的结合常数、结合位点数及热力学参数等。结果表明3, 3’-二硒苯二甲酸与HSA 1:1 结合,其反应主要是熵趋动的,相互作用的主要作用力为静电力。根据F?rster无辐射能量转移理论计算了给体(HSA)与 授体(3, 3’-二硒苯二甲酸)之间的结合距离。为有机硒化合物的开发与应用提供了重要信息。
关键词 荧光猝灭,3, 3’-二硒苯二甲酸,人血清白蛋白,热力学参数