http://www.chemistrymag.org/cji/2008/10a048pe.htm |
Oct.
1, 2008 Vol.10 No.10 P.48 Copyright |
Fu Min, Chen
Yao, Hu Jinghua
(Department of Chemistry, Jinan University, Guangzhou, 510632,China)
Supported by Foundation of Ministry of Education (No.2002247), Foundation of Guangdong
province (No.2003C33101) and Foundation of Jinan University (No.640071).
Abstract The experimental tie line data for five
ternary mixtures of water + ethanol +diethyl carbonate, water + methanol +diethyl
carbonate, water +diethyl carbonate + n-heptane, water + methyl tert-butyl
ether + n-heptane, water + methyl tert-butyl ether + diethyl carbonate have
been measured at temperature 298.15 K and ambient pressure. The experimental data were
correlated by using a modified UNIQUAC model. Furthermore, the calculated results were
compared with those obtained from an extended UNIQUAC model.
Keywords Liquid-liquid equilibria, Fuel additives, Ternary mixtures, Modified
UNIQUAC and extended UNIQUAC models.
1. INTRODUCTION
Presently, several fuel oxygenates are added to gasoline to enhance the octane number and
reduce air pollution. Methyl tert-butyl ether (MTBE) is currently the primary
oxygenated compound being used in lead-free gasoline. Diethyl carbonate (DEC) is
recognized as an environmentally benign chemical because of its negligible ecotoxicity and
low bioaccumulation and persistence. Its high oxygen content makes it a promising
candidate to be used as an oxygenated fuel additive, which has been reported by many
researchers [1-3]. Engine texts show that DEC reduces particulate emissions
almost 50% when the engine is under load. Without load, particulate emissions are reduced
by approximately 30% [4].
To assess the effect of the additives in gasoline reformulation, we
need a fundamental knowledge about multi-component phase equilibria of the mixtures
containing these compounds. In this work, we report liquid-liquid equilibria (LLE) data
for ternary mixtures of water + ethanol + DEC, water + methanol + DEC, water + DEC + n-heptane,
water + MTBE + n-heptane, water + MTBE + DEC systems measured at 298.15 K. The
experimental results were correlated by means of the extended and modified UNIQUAC models [5,
6] which include additional ternary parameters
arising from multicomponent intermolecular interactions, in addition to the binary
parameters. The vapor-liquid equilibria (VLE) data and mutual solubilities of the
constituent binary mixtures have been available from the literatures [7-14],
the mutual solubilities of water + DEC was measured in this work.
2. EXPERIMENTS
2.1 Materials
DEC was obtained from Alfa Aesar Company, with mass fraction of 0.990. MTBE was supplied
by Tedia Company, Inc. with mass fraction of 0.998. Methanol and n-heptane were
provided from Guangzhou Chemical Reagent Factory, with mass fraction of 0.995 and 0.997,
respectively. Ethanol was supplied from Tianjin Chemical Reagent Factory, with mass
fraction of 0.997. Water provided from Jinan University was distilled twice and had a mass
fraction purity of 0.999. The g.c. analysis gave mass fractions purities of 0.991 for DEC,
0.997 for MTBE, 0.995 for methanol, 0.996 for n-heptane, and
0.996 for ethanol. All chemicals were used directly in this work.
2.2 Apparatus and Procedures
Ternary LLE measurements were carried out at the
temperature (298.15 ± 0.01) K. The experimental apparatus was the same that reported in
detail previously [15]. The mixtures were stirred by using a magnetic stirrer
for 3 hours, and settled for 2 hours. It is sufficient to separate into two phases. The
samples withdrawn from upper and lower phases were analyzed by a gas chromatography. The
accuracy of the measurements was estimated within ±0.001 in mole fraction. Table 1 shows
experimental LLE results for the mixtures of water + ethanol + DEC, water + methanol +
DEC, water + DEC + n-heptane, water + MTBE + n-heptane, and water + MTBE +
DEC.
Table 1 Equilibrium phase compositions in mole fraction (x) for the five ternary mixtures at 25
ºCorganic phase |
|
||||
x1 |
x2 |
x3 |
x1 |
x2 |
x3 |
x1 water + x2 ethanol + x3 DEC |
|||||
0.0474 |
0.0000 |
0.9526 |
0.9978 |
0.0000 |
0.0022 |
0.0506 |
0.0874 |
0.8620 |
0.9491 |
0.0467 |
0.0042 |
0.0901 |
0.1502 |
0.7597 |
0.9123 |
0.0816 |
0.0061 |
0.1616 |
0.2242 |
0.6142 |
0.8695 |
0.1226 |
0.0079 |
0.2130 |
0.2846 |
0.5024 |
0.8275 |
0.1581 |
0.0144 |
0.2880 |
0.3345 |
0.3775 |
0.7865 |
0.1901 |
0.0234 |
0.3851 |
0.3521 |
0.2628 |
0.7258 |
0.2268 |
0.0474 |
x1 water + x2 methanol + x3 DEC |
|||||
0.0474 |
0.0000 |
0.9526 |
0.9978 |
0.0000 |
0.0022 |
0.0629 |
0.0199 |
0.9172 |
0.9332 |
0.0624 |
0.0044 |
0.0852 |
0.0560 |
0.8588 |
0.8801 |
0.1135 |
0.0064 |
0.1023 |
0.0912 |
0.8065 |
0.8426 |
0.1503 |
0.0071 |
0.1230 |
0.1303 |
0.7467 |
0.7947 |
0.1952 |
0.0101 |
0.1403 |
0.1858 |
0.6739 |
0.7571 |
0.2241 |
0.0188 |
0.1750 |
0.2271 |
0.5979 |
0.7215 |
0.2523 |
0.0262 |
0.2038 |
0.2590 |
0.5372 |
0.6885 |
0.2728 |
0.0387 |
x1 water + x2 DEC + x3 n-heptane |
|||||
0.0474 |
0.9526 |
0.0000 |
0.9978 |
0.0022 |
0.0000 |
0.0378 |
0.8715 |
0.0907 |
0.9989 |
0.0010 |
0.0001 |
0.0365 |
0.8271 |
0.1364 |
0.9993 |
0.0006 |
0.0001 |
0.0312 |
0.7955 |
0.1733 |
0.9995 |
0.0005 |
0.0000 |
0.0236 |
0.7394 |
0.2370 |
0.9996 |
0.0004 |
0.0000 |
0.0185 |
0.7014 |
0.2801 |
0.9998 |
0.0002 |
0.0000 |
0.0163 |
0.6425 |
0.3412 |
0.9998 |
0.0002 |
0.0000 |
0.0135 |
0.6069 |
0.3796 |
0.9999 |
0.0001 |
0.0000 |
0.0094 |
0.5456 |
0.4450 |
0.9999 |
0.0001 |
0.0000 |
0.0042 |
0.4811 |
0.5147 |
0.9999 |
0.0001 |
0.0000 |
0.0000 |
0.3976 |
0.6024 |
1.0000 |
0.0000 |
0.0000 |
0.0000 |
0.3174 |
0.6826 |
1.0000 |
0.0000 |
0.0000 |
0.0015 |
0.0000 |
0.9985 |
1.0000 |
0.0000 |
0.0000 |
x1 water + x2 MTBE + x3 n-heptane |
|||||
0.0572 |
0.9428 |
0.0000 |
0.9909 |
0.0091 |
0.0000 |
0.0456 |
0.8464 |
0.1080 |
0.9931 |
0.0067 |
0.0002 |
0.0315 |
0.7844 |
0.1841 |
0.9938 |
0.0062 |
0.0000 |
0.0290 |
0.7277 |
0.2433 |
0.9948 |
0.0052 |
0.0000 |
0.0237 |
0.6842 |
0.2921 |
0.9950 |
0.0050 |
0.0000 |
0.0199 |
0.6424 |
0.3377 |
0.9951 |
0.0049 |
0.0000 |
0.0209 |
0.5992 |
0.3799 |
0.9956 |
0.0044 |
0.0000 |
0.0116 |
0.5325 |
0.4559 |
0.9957 |
0.0043 |
0.0000 |
0.0108 |
0.4803 |
0.5089 |
0.9967 |
0.0033 |
0.0000 |
0.0073 |
0.4108 |
0.5819 |
0.9969 |
0.0031 |
0.0000 |
0.0053 |
0.3782 |
0.6165 |
0.9972 |
0.0028 |
0.0000 |
0.0028 |
0.3155 |
0.6817 |
0.9975 |
0.0025 |
0.0000 |
0.0000 |
0.2719 |
0.7281 |
0.9982 |
0.0018 |
0.0000 |
0.0015 |
0.0000 |
0.9985 |
1.0000 |
0.0000 |
0.0000 |
x1 water + x2 MTBE + x3 DEC |
|||||
0.0572 |
0.9428 |
0.0000 |
0.9909 |
0.0091 |
0.0000 |
0.0561 |
0.8629 |
0.0810 |
0.9915 |
0.0085 |
0.0000 |
0.0550 |
0.7738 |
0.1712 |
0.9921 |
0.0072 |
0.0007 |
0.0542 |
0.7255 |
0.2203 |
0.9929 |
0.0062 |
0.0009 |
0.0525 |
0.5853 |
0.3622 |
0.9937 |
0.0053 |
0.0010 |
0.0511 |
0.5165 |
0.4324 |
0.9943 |
0.0044 |
0.0013 |
0.0493 |
0.4270 |
0.5237 |
0.9945 |
0.0037 |
0.0018 |
0.0468 |
0.3634 |
0.5898 |
0.9946 |
0.0034 |
0.0020 |
0.0349 |
0.2981 |
0.6670 |
0.9948 |
0.0030 |
0.0022 |
0.0334 |
0.2050 |
0.7616 |
0.9943 |
0.0022 |
0.0035 |
0.0474 |
0.0000 |
0.9526 |
0.9978 |
0.0000 |
0.0022 |
3. CALCULATION PROCEDURE
The extended and modified UNIQUAC models which were described in the references 5 and 6,
were used to correlate the experimental ternary LLE data.
The binary energy parameters for the miscible mixtures were obtained
from the VLE data reduction using the following thermodynamic equations by using the
computer program [16]:
![]() (1)
(1)
![]() (2)
(2)
where P, x, y, and γ
Ternary parameters τ231, τ312, and τ123 were obtained by fitting the two models to the ternary LLE data using a simplex method [19] by minimizing the objective function:
F=
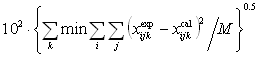 (5)
(5)where min denotes minimum values, i = 1 to 3 for ternary mixtures, j = 1, 2 (phases), k = 1, 2, …,M (number of tie lines), M = 2ni, and x is the liquid-phase mole fraction.
4. CALCULATED RESULTS
Table 2 lists the binary energy parameters of the modified and extended UNIQUAC models
for the constituent binary mixtures, along with the root-mean-square deviations between
experimental and calculated values: sP for pressure,
Table 3 gives the ternary parameters and predicted and correlated results for the ternary mixtures by using the modified and extended UNIQUAC models, along with the root mean square deviation between the experimental and calculated tie-lines for the ternary mixtures. The correlated results obtained with the models by using binary and ternary parameters are better than the predicted ones with only binary parameters. Figure 1 compares respectively the experimental LLE data and calculated results of modified UNIQUAC model for the mixtures water + ethanol + DEC, water + methanol + DEC, water + DEC + n-heptane, water + MTBE + n-heptane, and water + MTBE + DEC. The figure shows good agreement between ternary experiment LLE results and correlated results. The models can give an accurate representation for the ternary LLE by including the ternary parameters in addition to the binary ones. The LLE calculated results obtained by the modified UNIQUAC model give better agreement with the experimental results than these obtained by extended UNIQUAC.
Table 2 The results of fitting both models to the binary phase equilibria data
Mixture |
T/K |
Model |
a12/K |
a21/K |
σP/mmHg |
σT/K |
103σx |
103σy |
ethanol+DEC |
351.73–396.02 |
I |
39.99 |
314.36 |
1.6 |
0.1 |
3.0 |
8.9 |
ethanol+water |
298.15 |
I |
212.17 |
–46.98 |
0.1 |
0.0 |
1.5 |
6.0 |
methanol+DEC |
337.98–392.43 |
I |
–121.53 |
583.84 |
2.0 |
0.1 |
3.1 |
3.5 |
methanol+water |
298.14 |
I |
–160.39 |
158.59 |
0.6 |
0.0 |
0.6 |
4.0 |
DEC+ n-heptane |
371.49–398.17 |
I |
31.27 |
144.16 |
1.3 |
0.1 |
0.8 |
8.0 |
DEC+MTBE |
298.15 |
I |
–153.95 |
298.64 |
1.7 |
0.0 |
0.4 |
6.0 |
MTBE+ n-heptane |
326.45–366.45 |
I |
171.74 |
–100.25 |
2.2 |
0.1 |
1.8 |
5.7 |
water+DEC |
298.15 |
I |
248.21 |
1177.6 |
||||
water+n-heptane |
298.15 |
I |
1022.10 |
1884.20 |
||||
water+MTBE |
298.15 |
I |
173.24 |
1196.10 |
I, modified UNIQUAC model.
II, extended UNIQUAC model.
Table 3 The results of fitting both models to the ternary LLE data at 25
ºCMixture |
Na |
Ternary parameters |
Deviationsd |
||
Ib |
IIc |
Ib |
IIc |
||
water + ethanol + DEC |
7 |
t231 = –0.0233 t132 = 0.1227 t123 = 0.5686 |
t231=
–0.6855 t132= 1.7172 t123 = –1.4479 |
0.78e |
1.11 |
water + methanol +DEC |
8 |
t231
= –1.4775 t132 = 1.3433 t123 = –0.6417 |
t231
= –1.2812 t132 = 1.2728 t123 = –2.3477 |
0.65 |
1.36 |
water +DEC + n-heptane |
13 |
t231
= –0.0333 t132 = 0.1576 t123 = –0.0175 |
t231
= –0.0230 t132 = 0.1452 t123 = –0.8741 |
0.26 |
0.85 |
water + MTBE + n-heptane |
14 |
t231
= 0.0011 t132 = –0.0301 t123 = –0.1015 |
t231 = 0.0010 t132 = –0.9555 t123 = –0.0105 |
0.31 |
0.32 |
water + MTBE + DEC |
11 |
t231
= 0.0096 t132 = –0.1058 t123 = 0.1039 |
t231
= –0.0636 t132 = 0.1367 t123 = 0.1299 |
0.32 |
0.74 |
a
N, no. of tie-lines.b I, modified UNIQUAC model.
c II, extended UNIQUAC model.
d Root-mean-square deviations (mol%).
e Correlated results using binary and ternary parameters.
f Predicted results using binary parameters alone.
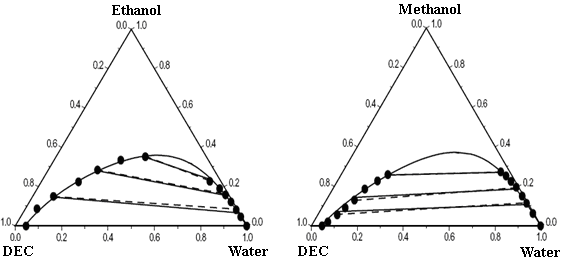
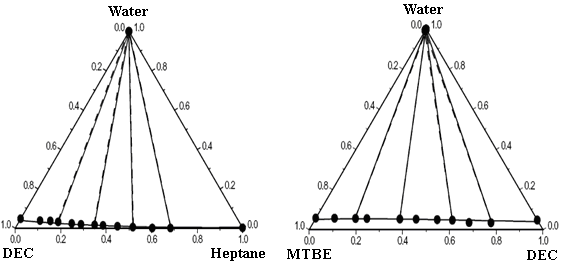
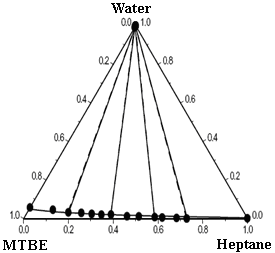
Figure 1 Experimental and calculated LLE of five ternary mixtures at 25ºC.
●- - -●, experimental tie line; ——, correlated by the modified UNIQUAC model.
5. CONCLUSIONS
The experimental tie line data were measured for five ternary systems of water +
ethanol + DEC, water + methanol + DEC, water + DEC + n-heptane, water + MTBE + n-heptane,
water + MTBE + DEC at 298.15 K. The correlated results using the modified UNIQUAC model
for the experimental ternary LLE data are much better than those obtained from the
extended UNIQUAC model.
REFERENCES
[1] Eyring E M, Meuzelaar H L C, Pugmire R J. Annual Report for Cooperative Research
in C1 Chemistry (DF-FC26-99FT40540), 2001: 32–36.
[2] Pacheco M A, Marshall C L. Energy Fuels, 1997, 11: 2–29.
[3] Dillon D M. U.S. Patent 4,891,049, Jan. 2, 1990.
[4] Dunn B C, Guenneau C, Hilton S A, et al. Energy Fuels, 2002,16: 177–181.
[5] Nagata I. Fluid Phase Equilib, 1990, 54: 191–206.
[6] Tamura K, Chen Y, Tada K, et al. J Solution Chem, 2000, 29: 463–488.
[7] Rodríguez A, Canosa J, Domínguez A, et al. J Chem Eng Data, 2003, 48: 86–91.
[8] Francesconi R.
J Chem Eng Data, 1997, 42: 697–701.
[9] Wisniak J, Magen E, Shachar M, et al. J Chem Eng
Data, 1997, 42: 243–247.
[10] Rodríguez A, Canosa J, Domínguez A, et al. J Chem Eng Data, 2002, 47:
1098–1102.
[11] Arce A, Blanco M, Riveiro R, et al. Can J Chem Eng, 1996, 74: 419–422.
[12] Sφrensen J M, Arlt W. Liquid–Liquid Equilibrium Data Collection, Vol. V, Part 1, DECHEMA,
Frankfurt am Main, 1979.
[13] Hall D J, Mash C J, Penberton R C. NPL Report Chem, 1979, 95: 1–32.
[14] Kooner Z S, Phutela, R C, Fenby D V. Aust J Chem, 1980, 33: 9–13.
[15] Chen Y, Dong Y H, Pan Z J. J Chem Thermodyn, 2005, 37: 1138–1143.
[16] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculation for
Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria . Prentice-Hall: Englewood
Cliffs, NJ, 1980:19.
[17] Spencer C F, Danner R P. J Chem Eng Data, 1972, 17: 236–241.
[18] Hayden J G, O’Connell J P. J Ind Eng Chem
Process Des Dev, 1975, 14: 209–216.
[19] Nelder J A, Mead R. J Comput, 1965, 7: 308–313.
付敏,陈瑶,胡竟华
(暨南大学化学系,广州,510632)
国家教育部留学回国人员科研基金(No.2002247),广东省科技计划基金(No.2003C33101)和广州暨南大学科研基金(No.640071)。
摘要 在298.15K和常压下,测定了五个三元体系水+乙醇+碳酸二乙酯、水+甲醇+碳酸二乙酯、水+碳酸二乙酯+正庚烷、水+甲基叔丁基醚+正庚烷、水+甲基叔丁基醚+碳酸二乙酯的液液相平衡数据,并用modified UNIQUAC 模型关联了这些实验数据,计算结果进一步地与extended UNIQUAC模型的关联计算结果进行了比较。
关键词 液液平衡,燃油添加剂,三元混合物,modified UNIQUAC和extended UNIQUAC 模型