http://www.chemistrymag.org/cji/2008/10a050pe.htm |
Oct.
1, 2008 Vol.10 No.10 P.50 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Supported by the fund from the Natural Science Foundation of Hebei Province (No. B2007000146) and Plan Item of Hebei Province Science-Technology Department(No.06213014)
Abstract A novel method for the
preparation of dry chitosan macrospheres was suggested, which employed glycerol as
treatment agent. The facts obtained showed that the chitosan macrospheres scarely shrank
during drying after 30% glycerol solution treatment for 1h. Meanwhile b-galactosidase from Aspergillus oryzae
was studied to be immobilized on the dry chitosan macrospheres with and without treatment
of glycerol solution, the results showed that the former was more suitable to immobilize
enzyme than the latter.
Keywords chitosan macrospheres; b-galactosidase; glycerol; immobilization; glutaraldehyde
Application of solid-phase biocatalysts had become important during the last decades. Enzymes could be immobilized on various supports and by different methods. The properties of the immobilized biocatalysts were influenced by the characteristics of enzyme, support material and the immobilization method. A suitable support should have high affinity to proteins, reactive functional groups, hydrophilicity, mechanical stability and rigidity. Chitosan is a natural polyaminosaccharide which offered the above characteristics; therefore it is often used for enzyme immobilization [1, 2].
Chitosan macrospheres with size falling into millimetre range were often used to immobilize enzyme for practical purposes, and it was often prepared by a simple method—the precipitation method [3]. Unfortunately, the macrospheres obtained shrunk greatly during drying because a lot of water was lost, thus the wet chitosan was generally stored in aqueous solution, which would largely limit the industrial applications of chitosan. How to prevent its shrinkage during drying? No report was found to our knowledge.
In this paper, the strong and flexible chitosan macrospheres without shrinkage during drying were obtained with glycerol solution as treatment agent and b-galactosidase from Asp. oryzae was employed to be immobilized on the above support with glutaraldehyde as crosslinker. 2. EXPERIMENTAL SECTION
2.1 Apparatus and reagents
Ultraviolet Spectrotometer (T6 New Century), Vacuum Desiccator (DZ-6020), Digital pH Meter (PHS-3C) and Water Constant Temperature Oscillator (SHA-B) were used for the study. All the aqueous solutions were prepared by twice distilled water.
b-galactosidase from Aspergillus oryzae (11.2U/mg) was obtained from Sigma. O-nitrophenyl-b-D-galactopyranoside (ONPG) was from Sigma. Chitosan was purchased from Jinan Haidebei Marine Bioengineering Co.Ltd. Glutaraldehyde, glycerol and other reagents were all analytical grades.
2.2 Preparation of enzyme and substrate solution
0.05g of b-galactosidase was weighed and extracted in 100mL 0.1M sodium phosphate buffer (pH 6.0), then the enzyme solution was obtained and stored in the refrigerator for use.
The substrate solution was prepared by dissolving 0.0751g ONPG in twice distilled water and made up 50mL solution.
2.3 Preparation of Chitosan Macrospheres
1g of chitosan was accurately weighed and dissolved in acetic acid (2%, w/v) to form 5% solution (w/v), The viscous solution obtained was placed in a vacuum dryer to remove air bubbles, and then was sprayed drop-wise through a syringe into a gently stirred coagulation liquid containing 15% NaOH and ethanol in a volume ratio of 4:1 at a constant rate [4]. The obtained macrospheres were filtered and washed with distilled water until neutrality. Then the macrospheres were divided into two groups, one group was stored in a refrigerator at 4oC (support I) and the other was dried in the air (support II).
2.4 The chitosan macrospheres treated with glycerol solution
The chitosan macrospheres were obtained according to section 2.3, and then they were immersed in a 30% aqueous glycerol solution for 1 hour. After the excess glycerol solution was removed, the obtained chitosan macrospheres were divided into two groups. One group was washed with distilled water for three times and stored in a refrigerator for use (support III) and the other was dried in a vacuum desiccator for 8 hours at 85oC and then stored at room temperature for use (support IV).
2.5 Preparation of Activated Chitosan Macrospheres by Glutaraldehyde
2g of the chitosan macrospheres were washed and immersed in distilled water for 12h. After being dried with filter paper, the beads were placed in a 50-mL vessel containing 20mL of 0.1% glutaraldehyde (pH 8.0), and crosslinking was carried out by shaking it for 2h at 30oC, then the activated macrospheres obtained were rinsed thoroughly and stored for the next use.
2.6 Immobilization of b-galactosidase on Activated Chitosan Macrospheres
The immobilization was carried out by adding an amount of activated macrospheres (0.1g) to 0.1M phosphate buffer (pH 6.0) containing enzyme (0.5mg/mL). With gently stirring, the reaction was allowed to proceed at 4oC, after 16 hours, the immobilized enzyme was filtered and washed with 0.1M phosphate buffer (pH 6.0) until no protein was detected.
2.7 Determination of Enzyme Activity
The activity of the free and the immobilized enzyme were determined according to the references [5, 6] using ONPG as a substrate. For the free enzyme activity, aliquots of it (0.1 mL) were added to the mixture of 1.8 mL 0.1M phosphate buffer (pH 6.0) and 0.1 ml ONPG (5mM), after being incubated at 55oC for 15min, the reaction of ONPG was stopped by the addition of 2 mL 1 mol/L Na2CO3 solution, and the amount of ONP was measured directly at 405 nm. For the immobilized enzyme activity, 0.5 g of the immobilized enzyme was soaked in 1.9mL 0.1M phosphate buffer, the reaction was started by adding 0.1mL ONPG (5mM). After being carried out for 15min at 55oC, the reaction was stopped and analyzed as above. The activity yield was calculated as the ratio of immobilized enzyme to enzyme subjected to immobilization. One unit of activity was defined as the amount of enzyme that liberated 1mmol of product/min at 55oC. 3. RESULTS AND DISCUSSION
3.1 Discussion of Glycerol Concentration and Treatment Time
It was found that the chitosan macrospheres shrunk greatly during drying (about from 2.2mm to 0.9mm), and the diameter of the dry macrospheres was nearly fixed after being immersed in distilled water or buffer solution for enough time, which meant the structure of the chitosan macrospheres was destroyed during drying. Another experiment was also carried out to test above viewpoint; both wet and dry chitosan particles were taken and used to immobilize b-galactosidase under the same conditions, the enzyme activity of the latter was far lower than the former. Therefore, the wet chitosan macrospheres must be treated further before drying.
In our experiments, glycerol solution was selected as treatment agent, and the effects of glycerol concentration and treatment time on the chitosan macrospheres were also investigated. Firstly, the treatment time of glycerol solution ranging from 0.5 to 2.0 h was discussed and the dry chitosan macrospheres treated by above solution were used to immobilize b-galactosidase, the results obtained were presented in Fig. 1. From Fig.1, it could be seen that the enzyme activity of the immobilized enzyme increased firstly then decreased with the increase of treatment time and 1.0 h was found to be the best based on the highest enzyme activity. Then, various concentrations of glycerol solution from 10% to 60% were also examined, the dry carrier obtained was also used to bound enzyme under the same conditions and the results were shown in Fig.2. From Fig.2, it could be seen that the activity of the immobilized enzyme was the highest when the glycerol concentration was 30%, which might be explained by the following facts, the structure of the chitosan macrospheres could not be kept if the glycerol concentration was lower and the hole of the carrier could be blocked up if the concentration of the treatment solution was much higher. So 30% glycerol solution was selected in the next experiments.
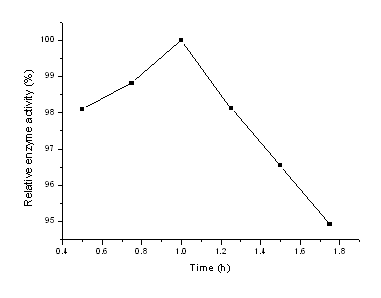
Fig. 1 Effect of glycerol treatment time on the chitosan macrospheres
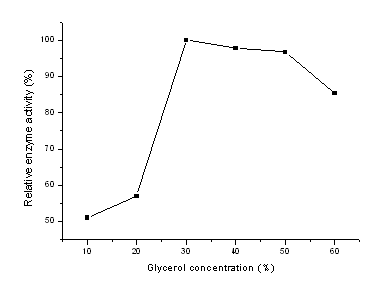
Fig. 2 Effect of glycerol concentration on the chitosan macrospheres
3.2 Effect of Glycerol Solution on chitosan
macrospheres
In order to show the effect of the glycerol solution on the chitosan macrospheres, four
kinds of supports were taken and employed to immobilize enzyme, the results obtained were
listed in Table 3-1. From Table 3-1, it could be seen that the enzyme activity of the wet
macrospheres, the wet with glycerol treatment and the dried with glycerol treatment were
almost the same, however, the enzyme activity of the dried support without glycerol
treatment was just 10.29% of the wet support. Based on above facts, we could see that
chitosan macrospheres could be prevented shrinkage with glycerol treatment and kept nearly
the same structure as its wet form. In the references reported using chitosan as
immobilization carrier [7, 8], wet macrospheres often employed in order to
avoid its shrinkage during dying. It was not very good because the storage of wet form was
inconvenient. Chitosan was also coupled with other materials [9], which could
prevent chitosan macrospheres from shrinking, but materials obtained were complex and the
reaction process was usually very complicated. So the method developed here was very
important for chitosan as enzyme immobilization carrier in industry.
Table 1 Comparison of immobilized enzyme activity of different chitosan supports
Type of supports |
Immobilized enzyme activity (U/g dry support) |
I (the wet support) |
71.43 |
II ( the dried support without glycerol treatment) |
7.35 |
III (the wet support with glycerol treatment) |
71.29 |
IV (the dried support with glycerol treatment) |
69.61 |
3.3 Preparation of Activated Chitosan
Macrospheres by Glutaraldehyde
Three factors (the concentration of glutaraldehyde, the coupling pH, and reaction time)
were investigated during the preparation of the activated carrier. Firstly, the effect of
glutaraldehyde concentration on the activated carrier was studied, and the results showed
that the immobilized enzyme activity increased firstly then decreased with the increase of
the concentration of the glutaraldehyde, when the concentration of the glutaraldehyde was
0.1% (0.14mL/mg dried carrier), the immobilized enzyme activity attained to the maximum.
The phenomena above might be explained by the following facts: with the increase of the
concentration of the glutaraldehyde, the activated group was increased, which could
enhance the immobilization of the enzyme and the activity of the immobilized enzyme was
increased as well. However, a further increase in glutaraldehyde concentration resulted in
decrease of the enzyme activity. This might be due to that the active center of the enzyme
was affected because the enzyme was bound on the support in multipoint way with increase
of glutaraldehyde concentration, thus the activity of the immobilized enzyme was decreased
accordingly. Secondly, the influence of pH on the preparation of the activated support was
investigated and the optimal pH was determined to be 8.0. Finally, the activated reaction
time was also studied in our experiments; the results showed that 2h was the best choice
in the preparation of activated carrier.
3.4 Immobilization of b-galactosidase on Activated Chitosan Macrospheres
In this part, the effects of enzyme concentration, coupling pH and reaction time were
studied. Firstly, various enzyme concentrations were added into the reaction system, and
the results determined showed that the enzyme activity increased at first, then kept
nearly constant. 0.5mL enzyme solution(0.5mg/mL-1)per 7mg dry carrier was taken in the later investigation
considering both higher enzyme activity and activity yield. Secondly, the influence of pH
on the immobilization of b-galactosidase
was studied in the range of 5.0–9.0, and the
activity of the immobilized enzyme was the highest at pH 6.0. Finally, various coupling
time from 12h to 24h was investigated at 4oC and the results showed 16h was the best selection based on the
highest enzyme activity in our reaction system.
Under the optimal conditions obtained above, b-galactosidase was immobilized on the dry chitosan macrospheres
with glycerol solution treatment and without treatment, the activity of the immobilized
enzyme and the activity yield for the former was 69.61U/g dry carrier and 60.35%
separately, and the value for the latter was 7.35U/g dry carrier and 5.97% respectively.
Therefore, it was concluded that chitosan macrospheres treated with glycerol solution were
more appropriate for enzyme immobilization.
In this work, a new method for the preparation of dry chitosan macrospheres was developed by treating with glycerol, and it was proved by the experiments that the chitosan macrospheres scarcely shrank during drying after glycerol treatment. Meanwhile, b-galactosidase from Aspergillus oryzae was studied to be immobilized on above support. The results showed that the activity of the immobilized enzyme on dry chitosan macrospheres with glycerol treatment was nearly the same as that on the wet support, while a poor activity of the immobilized enzyme on dry chitosan macrospheres without glycerol treatment was found. Therefore, dry chitosan macrosphere with glycerol treatment could be kept nearly the same structure as its wet one. Considering the inconvenience of the storage of the wet form, the dry chitosan macrospheres were more applicable.
The conclusion obtained here was very useful for industrial application of chitosan macrospheres as enzyme immobilization carrier, and the procedure developed were simple, fast and inexpensive.
REFERENCES
[1] Jaafar A , Musa A , Nadarajah K. et al. Sensors and Actuators B:
Chemical, 2006, 114 (2) : 604.
[2] Gamze DA,Senay AC, Food Chemistry, 2007, 100 (3) : 964.
[3] Chiu S H, Chung T W, Giridhar R et al. Food Research International, 2004, 37:217.
[4] Chang M Y, Juang R S. Biochemical Engineering Journal, 2007, 35: 93.
[5] Tu W X, Sun S F, Nu S L, et al. Food Chemistry, 1998, 64: 1.
[6] Sun S F, Li X Y, Nu S L, et al. J. Agric. Food Chemistry, 1999, 47: 819.
[7] Emese B, Agnes S N, Csaba S. et al. Biochem. Biophys. Methods, 2008, 70 (6): 1240.
[8] Dey C S, Shin Y L, Kow J D. et al. Biotechnology Techniques, 1998, 12(4): 273.
[9] Xi F N, Wu J M. Reactive and Functional Polymers, 2006, 66 (6): 682.
甘油后处理壳聚糖微球固定化米曲霉b-半乳糖苷酶的研究
孙素芳* 张燕
董凌云 吕树芳
(河北大学化学与环境科学学院
保定071002)
摘要
本文首次采用甘油对湿润壳聚糖微球进行了后处理,避免了微球在干燥过程中的严重缩孔现象,得到了干燥的壳聚糖微球固定化酶载体。湿润壳聚糖微球经30%甘油处理1小时后进行干燥,没有明显的体积收缩现象,同时我们分别以不同状态的壳聚糖微球为载体,进行了米曲霉b-半乳糖苷酶的固定化,结果表明:甘油处理后干燥的壳聚糖微球能够保持其湿态时的结构,更适合酶的固定化。
关键词 壳聚糖微球;b-半乳糖苷酶;甘油;固定化;戊二醛