http://www.chemistrymag.org/cji/2009/111001pe.htm |
Jan.1,
2009 Vol.11 No.1 P.1 Copyright |
Facile synthesis of 3,3-di(indolyl)indolin-2-one derivatives catalyzed by ZrO2/S2O82- solid superacid under grinding condition
Feng Guoliang, Geng Lijun, Zhang Hongli
(School of Science, Hebei University of Science and Technology, Shijiazhuang 050018)
Keywords 3,3-di(indolyl)indolin-2-ones, ZrO2/S2O82- solid superacid, isatin, indoles, grinding
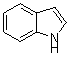
The indole moiety is probably the most
well-known heterocycle, a common and important feature of a variety of natural products,[1]
as well as in many compounds that show pharmacological and biological activities.[2-4]
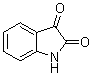
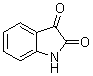
Oxindole derivatives are known to possess a bariety of bilolgical activity.[5,6]
The 3,3-diaryloxindoles have been shown to possess mechanism-specific antiproliferative,
antibacterial, antiprotozoal, and antiinflammatory activies.[7] In view of the
biological importance of 3,3-di(indolyl)indolin-2-ones, several methods for their
synthesis have been reported. The classical method involves the reaction of isatin and
indoles in acid conditions for long reaction times or promoted by KAl(SO4)2
under microwave conditions.[8-10] Some of these methods have not been entirely
satisfactory owing to such drawbacks as long reaction times, cumbersome experimental and
non-recoverable catalysts. Therefore, the search for a better method for the synthesis of
3,3-di(indolyl)indolin-2-ones is still the need of the day.
In recent times, the progress in the field of solvent-free reactions is
gaining significance because of their high efficiency, operational simplicity and
environmentally benign processes. Solventless organic reactions based on grinding have
been investigated for the intensive subject in recent years. The grinding mode for the
solid-state reactions has earlier been reported for Grignard reaction, Reformatsky
reaction, Aldol condensation, Dieckmann condensation, Knoevenagel condensation, reduction,
etc.[11-14] Most of these reactions are carried out at room temperature in
absolutely solvent-free environment using only a mortar and pestle. Solid superacid have
received considerable attention as powerful reaction media for effecting various
transformations.[15,16] In addition solid superacids are attractive because
they are stable, reusable, green and cheap. Recently, we have developed efficient and
convenient procedures for the preparation of some organic compounds catalysed by ZrO2/S2O82-
solid superacid.[17,18] As a part of ongoing work on grinding and solid
superacid catalysis, we now describe a facile and solvent-free, simple and practical
method for synthesis 3,3-di(indolyl)indolin-2-ones from isatin with indoles catalyzed by
ZrO2/S2O82- solid superacid under solvent-free
conditions at room temperature on grinding, which provide an efficient route to the
synthesis of symmetrical 3,3-di(indolyl)indolin-2-ones derivatives (Scheme 1).
The optimized procedure for reaction for indole to isatin was found to
be as follows: the mixture of isatin (1 mmol), indole (2.1 mmol) and ZrO2/S2O82-
(20 mg) was grinded at room temperature. Representative results of this study are
summarized in Table 1. As shown in Table 1, isatin in the presence of ZrO2/S2O82-
were grinded with indoles in free solvent, the corresponding
3,3-di(indolyl)indolin-2-ones were obtained in good to excellent yield. The reaction
proceeded cleanly and work-up was simple, involving only filtration of the catalyst to
obtain the product.
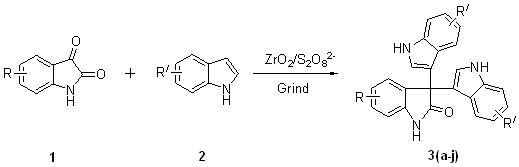
Scheme 1
Table 1 The reaction of isatin with indoles catalyzed by ZrO2/S2O82- under grinding
Entry |
Isatins |
Indoles |
Products |
Time(min) |
Yieldsa(%) |
1 |
|
|
3a |
8 |
89 |
2 |
|
|
3b |
8 |
90 |
3 |
|
|
3c |
8 |
87 |
4 |
|
|
3d |
8 |
85 |
5 |
|
|
3e |
8 |
89 |
6 |
|
|
3f |
8 |
86 |
7 |
|
|
3g |
12 |
76 |
8 |
|
|
3h |
8 |
90 |
9 |
|
|
3i |
8 |
85 |
10 |
|
|
3j |
8 |
87 |
aYield of pure isolated products.
We were pleased to find
that the conversion rate of the indoles bearing electron-withdrawing group (5-nitro-1H-indole)
provided lower conversion rate (especially for 7-nitro-1H-indole, no reaction) than
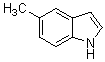
the indoles bearing donate group (5-methyl-1H-indole, 6-methyl-1H-indole,
7-methyl-1H-indole and 5-(benzyloxy)-1H-indole), this indicated that
electron-donating groups had increased reaction yields. On the other hand
electron-withdrawing groups, which deactivated the indole ring, had decreased yields. This
reaction also tolerated different substituents such as bromo and methyl group at the ring
in isatin.
It is known that the 3-position of the indole is the preferred site for
electrophilic attack. In the presence of ZrO2/S2O82-,
the electrophilic attack uniquely occurred at the 3-position of the indole ring. As
anticipated if the 3-position was occupied, the reaction could not proceed. In our
experiments, 3-carbaldehyde indole and 3-cyanoindole were used for the reaction, they all
remained intact with isatin under given condition even after 30 minutes.
It should be noted that in the absence of catalyst lower yields of
product were observed even with prolonged reaction time. For example, entry 2 without
catalyst after 20 minutes 32% yield of product was obtained, whereas 90% yield was
obtained with catalyst for 8 minutes. The catalyst was easily regenerated by washing with
ethanol followed by drying at 100
In summary, we have developed a practical and efficient method for the synthesis of 3,3-di(indolyl)indolin-2-ones under solvent-free conditions using isatin and indoles in the presene of ZrO2/S2O82-. This method is superior from the view of operation simplicity, high yields, non-corrosion, and friendliness to the environment than previously reported methods.
Melting points were determined by an XT-5A micro-melting point apparatus and are uncorrected. 1H NMR spectra were determined on a Varian VXP-400s spectrometer using CDCl3 or DMSO as solvent and tetramethylsilane (TMS) as internal reference. IR Spectra was obtained on a Nicolet FT-IR500 spectrophotometer using KBr pellets. Elementary analyses were performed by a Carlo-Erba EA1110 CNNO-S analyzer. The catalyst ZrO2/S2O82- solid superacid was prepared as follows. Zr(OH)4 was infused in an aqueous (NH4)2S2O8 solution (1 mol/l) for 4 hours, then filtered off, dried at 110oC for 2 hours, crushed over 150 mesh, dried and calcined in a furnace at 600oC for 4 hours and finally stored in a desiccaor until used.
A mixture of isatin 1 (1 mmol), indole 2 (2.1 mmol) and ZrO2/S2O82- (20 mg) were ground by mortar and pestle at room temperature for 8 min, and was kept for a period. The process of the reaction was determined by TLC. After the completion of the reaction, the mixture was extracted with ethyl acetate (10 mL×3) and dried over MgSO4. Evaporation of the solvent under reduced pressure afforded the crude product. After column chromatography on silica gel eluting with PE : EA (5:1), pure compounds 3(a-j) were obtained.
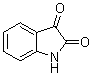
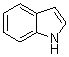
3,3-Di(1H-indol-3-yl)indolin-2-one(3a): m.p.: >300 oC; IR (KBr) n : 741, 1100, 1467, 1616, 1690, 3056, 3125, 3399, 3442 cm-1; 1H NMR (CDCl3, 400 MHz) d: 6.91-7.00 (m, 6H), 7.10-7.23 (m, 3H), 7.34-7.39 (m, 5H), 7.73 (s, 1H, NH), 8.09 (s, 2H, NH); Anal. Calcd. for C24H17N3O: C, 79.32; H, 4.72; N, 11.56. Found: C, 79.30; H, 4.75; N, 11.64.
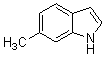
3,3-Di(5-methyl-1H-indol-3-yl)indolin-2-one(3b): m.p.: >300 oC; IR (KBr) n : 750, 1109, 1235, 1466, 1615, 1712, 2858, 2924, 3389, 3421 cm-1; 1H NMR (CDCl3, 400 MHz) d: 2.41 (s, 6H, CH3), 6.78 (d J=7.6 Hz, 2H), 6.92-7.01 (m, 5H), 7.12-7.38 (m, 5H), 7.53 (s, 1H, NH), 7.92 (s, 2H, NH); Calcd. for C26H21N3O: C, 79.77; H, 5.41; N, 10.73. Found: C, 79.59; H, 5.55; N, 10.64.
3,3-Di(6-methyl-1H-indol-3-yl)indolin-2-one(3c): m.p.: 296-298 oC; IR (KBr) n : 743, 1100, 1235, 1472, 1620, 1698, 2853, 2910, 3172, 3320, 3429 cm-1; 1H NMR (CDCl3, 400 MHz) d: 2.31 (s, 6H, CH3), 6.94-7.0 (m, 7H), 7.14-7.32 (m, 5H) 7.50 (s, 1H, NH), 7.96 (s, 2H, NH); Anal. Calcd. for C26H21N3O: C, 79.77; H, 5.41; N, 10.73. Found: C, 79.62; H, 5.50; N, 10.64.
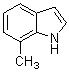
3,3-Di(7-methyl-1H-indol-3-yl)indolin-2-one(3d): m.p.: 197-198.5.oC; IR (KBr) n :750, 1100, 1343, 1472, 1617, 1700, 3054, 3413 cm-1; 1H NMR (CDCl3, 400 MHz) d: 2.45 (s, 6H, CH3), 6.85-6.98 (m, 8H), 7.18-7.23 (m, 3H), 7.40 (d, J=7.2 Hz, 1H), 7.72 (s, 1H, NH), 8.00 (s, 2H, NH); Anal. Calcd. for C26H21N3O: C, 79.77; H, 5.41; N, 10.73. Found: C, 79.72; H, 5.32; N, 10.84.
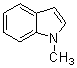
3,3-Di(1-methyl-1H-indol-3-yl)indolin-2-one(3e): m.p.: >300 oC; IR (KBr) n : 1207, 1245, 1455, 1618, 1693, 2874, 2939, 3057, 3320, 3438 cm-1; 1H NMR (CDCl3, 400 MHz) d: 3.70 (s, 6H, CH3), 6.85 (s, 2H), 6.88-7.40 (m, 12H), 7.76 (s, 1H, NH); Anal. Calcd. for C26H21N3O: C, 79.77; H, 5.41; N, 10.73. Found: C, 79.81; H, 5.40; N, 10.79.
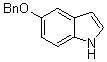
3,3-Di(5-(benzyloxy)-1H-indol-3-yl)indolin-2-one(3f): m.p.: 198-199.5 oC; IR (KBr) n : 744, 1100, 1382, 1469, 1480, 1699, 2914, 3027, 3385, 3423 cm-1; 1H NMR (CDCl3, 400 MHz,) d: 6.83-7.01 (m, 8H), 7.10-7.50 (m, 18H), 7.92 (s, 1H, NH), 7.98 (s, 2H, NH); Anal. Calcd. for C38H29N3O3: C, 79.28; H, 5.08; N, 7.30. Found: C, 79.41; H, 5.18; N, 7.39.
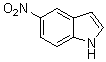
3,3-Di(5-nitro-1H-indol-3-yl)indolin-2-one(3g): m.p.: >300 oC; IR (KBr) n : 742, 1092, 1251, 1260, 1468, 1620, 1710, 3343 cm-1; 1H NMR (DMSO-d6, 400 MHz) d: 7.00-7.08 (m, 2H), 7.20-7.31 (m, 4H), 7.58 (d, J=8.0 Hz, 2H), 8.0 (d, J=8.0 Hz, 2H), 8.21 (s, 2H), 10.92 (s, 1H, NH), 11.80 (s, 2H, NH); Anal. Calcd. for C24H15N5O5: C, 63.58; H, 3.33; N, 15.45. Found: C, 63.44; H, 3.18; N, 15.37.
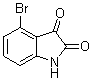
4-Bromo-3,3-di(1H-indol-3-yl)indolin-2-one(3h): m.p.: >300 oC; IR (KBr) n : 735, 1106, 1614, 1702, 3049, 3330, 3383 cm-1; 1H NMR (CDCl3, 400 MHz) d: 6.96 (d, J=7.0 Hz, 1H), 7.0-7.06 (m, 4H), 7.10-7.20 (m, 4H), 7.39 (d, J=8.0 Hz, 2H), 7.50 (d, J=9.2 Hz, 2H), 8.0 (s, 1H, NH), 8.10 (s, 2H, NH); Anal. Calcd. for C24H16BrN3O: C, 65.17; H, 3.65; N, 9.50. Found: C, 65.24; H, 3.55; N, 9.42.
6-Bromo-3,3-di(1H-indol-3-yl)indolin-2-one(3i): m.p.: >300 oC; IR (KBr) n : 741, 1117, 1449, 1605, 1709, 3027, 3112, 3398, 3440 cm-1; 1H NMR (DMSO-d6, 400 MHz) d: 6.80 (d, J=7.6 Hz, 2H), 6.82 (d, J=2.4 Hz, 2H), 7.0 (t, J=7.6 Hz, 2H), 7.10-7.15 (m, 3H), 7.19 (d, J=8.0 Hz, 2H), 7.32 (d, J=8.0 Hz, 2H), 10.72 (s, 1H, NH), 11.00 (s, 2H, NH); Anal. Calcd. for C24H16BrN3O: C, 65.17; H, 3.65; N, 9.50. Found: C, 65.34; H, 3.52; N, 9.45.
3,3-Di(1H-indol-3-yl)-1-methylindolin-2-one(3j): m.p.: >300 oC; IR (KBr) n : 743, 1092, 1350, 1613, 1668, 2925, 3043, 3116, 3352, 3440 cm-1; 1H NMR (CDCl3, 400 MHz) d: 3.34 (s, 3H, CH3), 6.90-7.05 (m, 6H), 7.10 (t, J=7.2 Hz, 3H), 7.32 (t, J=6.8 Hz, 4H), 7.43 (d, J=7.2 Hz, 1H), 8.02 (s, 2H, NH); Anal. Calcd. for C25H19N3O: C, 79.55; H, 5.07; N, 11.13. Found: C, 79.42; H, 5.22; N, 11.25.
REFERNECES
[1] Liu Y, Gribble G W, Tetrahedron Lett. 2000, 41: 8717.
[2] Walsh T F, Toupence R B, Ujjainwalla F, Young J R, Goulet M T, Tetrahedron, 2001, 57:
5233.
[3] Zhang H C, Ye H, Moretto A F, Brumfield K K, Maryanoff B E, Org. Lett. 2000, 2: 89.
[4] Russell M G N, Baker R J, Barden L, et al. J. Med. Chem. 2001, 44: 3881.
[5] Klumpp D A, Yeung K Y, Prakash G K S, et al. J. Org. Chem. 1998, 63: 4481.
[6] Gazit A, Osherov N, Posner I, et al. J. Med. Chem. 1991, 34: 1896.
[7] Pajouhesh H, Parsons R, Popp F D, J. Pharm. Sci. 1983, 72: 318.
[8] Wang SY, Ji S J, Tetrahedron, 2006, 62: 1527.
[9]Yadav J S, Subbareddy B V S, Gayathri K U, Meraj S, Prasad A R, Synthesis, 2006,
24: 4121.
[10] Chakrabarty M, Mukherjee R, Mukherji A, Arima S, Harigaya Y, Heterocyles,
2006, 68: 1659.
[11] Tabaka K, Kishigami S, Toda F, J. Org. Chem. 1991, 56: 4333.
[12] Toda F, Tanaka K, Hamai K, J. Chem. Soc., Perkin Trans. 1 1990, 3207.
[13] Wang S X, Li J T, Geng L J, J. Chem. Research (S), 2003, 370.
[14] Ren Z J, Cao W G, Ding W Y, Shi W, Synth. Commun. 2004, 34: 4395.
[15] Hino M, Arata K, J. Chem. Soc. Chem. Commun. 1979, 101: 1148.
[16] Hino M, Kobayashi S, Arata K, J. Am. Chem. Soc. 1979, 101: 6439.
[17] Jin T S, Feng G L, Yang M N, Li T S. Synth. Commun. 2004, 34: 1277.
[18] Jin T S, Yang M N, Feng G L, Li T S, J. Chem. Research (S), 2003, 11: 721.
固体超强酸ZrO2/S2O82-催化无溶剂研磨条件下对称3,3-二(吲哚基)吲哚-2-酮的合成
冯国良, 耿丽君,张红利
(河北科技大学理学院,石家庄 050018)
摘要 研究了固体超强酸ZrO2/S2O82-催化、无溶剂研磨条件下,对称3,3-二(吲哚基)吲哚-2-酮的合成反应。产物结构由红外光谱、核磁共振氢谱、及元素分析等确证。该方法具有无污染、高产率、操作简单、催化剂可回收重复利用等特点。
关键词 3,3-二(吲哚基)吲哚-2-酮, ZrO2/S2O82-
固体超强酸, 靛红, 吲哚,研磨