http://www.chemistrymag.org/cji/2009/111002pe.htm |
Jan.1,
2009 Vol.11 No.1 P.2 Copyright |
Qin Xinying1,
Lu Yunkai1,Zhang Puzhe2(1College of Chemistry and Environmental Science, Hebei University, Key Laboratoryo f Analytical Science and Technology of Hebei Province, Baoding 071002, China; 2Hebei Handan foreign Language School,Handan 056000,China)
Received Oct. 29,2007.
Abstract The kinetics and mechanism of
ruthenium(III) catalyzed oxidation of isobutyl alcohol(IBA) by cerium(IV) in sulfuric acid
media has been investigated by titrimetric technique of redox in the temperature range of
298-313K. It is found that the reaction is first-order with respect to Ce(IV) and Ru(III),
and a positive fractional order with respect to IBA. It is found that the pseudo
first-order ([IBA]>>[Ce(IV)]>>[Ru(III)])
rate constant kobs increases with the increase of [H+] but decreases
with the increase of [HSO4-]. Under the protection of nitrogen, the
reaction system can not initiate polymerization of acrylonitrile, indicating generation of
free radicals. On the basis of the experimental results, a suitable mechanism has been
proposed. The rate constants of the rate determining step together with the activation
parameters were evaluated.
Keywords Ruthenium(III) ion; Cerium(IV) ion; Isobutyl alcohol(IBA); Catalytic
Oxidization; Kinetics and mechanism
1. INTRODUCTION
Transition metals in a higher oxidation state generally can be stabilized by chelation
with suitable complex agent. Metal complexes such as silver (III)[1],
copper(III) [2] and Ce(IV) ions[3] are good oxidants in a medium
with an appropriate condition. However, our preliminary observations indicate that
oxidation of some organic compounds by Ce(IV) in aqueous sulfuric acid is kinetically
sluggish, the process can be efficiently catalyzed by various metal ions even at trace concentration. Different metal ion catalysts
like chromium(III)[4],
ruthenium(III)[5], iridium(III)[6] etc. have been used in the oxidation by
cerium(IV). Among the different metal ions, ruthenium(III) and iridium(III) are highly
efficient. In the modern chemical industry, the higher requirement on catalyst selectivity
has been advanced. However, the studies of active center structure by means of physical
method can not calculate reaction pathways about complicated molecules. Reaction mechanism of
various elementary reactions must be investigated to analyze the factors of affecting the
selectivity[7]. Therefore, the basic study of catalytic reaction will provide
the scientific basis for improving catalyst selectivity and making high-efficiency
catalyst. Now the lack of studying on the Ce(IV) oxidation of IBA stimulated us to
explore the kinetic behavior of the title reaction in order to continue our studying on
metal ion catalysis in cerium(IV) oxidation reactions.
2. EXPERIMENTAL
2.1 Materials and reagents
Ceric sulfate (Ce(SO4)2),
ferrous ammonium sulfate (Fe(NH4)2(SO4)2),
isobutyl alcohol(IBA) and ruthenium trichloride (RuCl3) were of A.R. grade.
Doubly distilled water was employed throughout the experiment. Ceric sulfate solution was
prepared by dissolving
2.2 Procedure and kinetic measurements
Under the condition of [IBA]>>[Ce(IV)]>>[Ru(III)], 25mL of solution containing definite [Ce(IV)], [Ru(III)], [H2SO4] and [NaNO3], and 25mL of IBA solution of appropriate concentration were transferred separately to the upper and lower branch tubes of l type two-cell reactor. After it was thermally equilibrated in a thermostat (about five minutes), the solutions were thoroughly mixed. The progress of the reaction was monitored by withdrawing aliquot of the reaction mixture at regular intervals, adding it into excess standard Fe(NH4)2(SO4)2 sulfate solution in sulfuric acid to quench the reaction, then back-titrating the unreacted iron(II) by standard cerium(IV) solution using ferroin as indicator. The pseudo first-order rate constants kobs were evaluated (Figure 1) from the slope of the plots of ln[Ce(IV)]t versus time (t) i.e. ln((V∞-Vt) versus t where V∞ and Vt denote the volume of standard cerium (IV) solution needed in back titration for the unconsumed iron(II) solution at infinity and time(t) respectively. To evaluate kobs, generally 8-10 values at least up to 80% completion of the reaction were used. Average values of at least two independent determinations of kobs were taken for analysis. The observed rate constants were reproducible within the experimental error±5%.
3. RESULTS AND DISCUSSION
3.1 Dependence on [Ce(IV)]
Under the condition [IBA]>>[Ce(IV)]>>[Ru(III)], the plot of ln (V∞-Vt) versus t was linear(Fig.1), indicating that the reaction is first-order with respect to [Ce(IV)]. The pseudo first-order rate constants kobs are independent upon the initial concentration of cerium (IV) in the 1.0~ 3.0×10-3mol·dm-3 range. The dependence is given by:
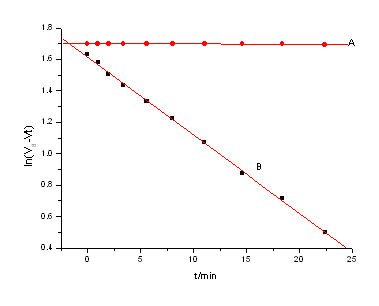
Fig.1 Plots of ln (V∞-Vt) vs. t at 298K
[Ce(IV)]=2.5×10-3 mol·dm-3, [IBA]=0.1 mol·dm-3, μ=1.0 mol·dm-3, [H2SO4]=1.0 mol·dm-3, r=0.9995
(A) [Ru(III)]=0.0 mol·dm-3, (B) [Ru(III)]=5.0×10-7 mol·dm-3
3.2 Dependence on [IBA]
At fixed [Ce(IV)], [Ru(III)], [H2SO4], ionic strength (m) and temperature (T), kobs increases
with the increase of [IBA]. The plot of kobs vs. [IBA] was a smooth curve,
indicating fractional order in [IBA] ,observed reaction order nap= 0.61(r=0.9988). The plot of 1/kobs
vs. 1/[IBA] exhibits excellent linearity (Fig.2) with a positive intercept and slope.
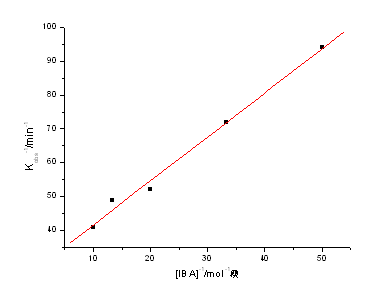
Fig.2 Plot of 1/kobs vs. 1/[IBA] at 30
[Ce(IV)]=2.5×10-3 mol·dm-3; [H2SO4]=1.0 mol·dm-3, [Ru(III) ]= 1×10-7 mol·dm-3; I=1.0 mol·dm-3
3.3 Dependence on [Ru(III)]
The fact that under the experimental conditions in the absence of Ru(III) ions the
reaction practically does not take place is supported by an independent experiment (Fig.1
(a)).Addition of traces of Ru(III) enhances the rate significantly (Fig.1 (b)), kobs
vs. [Ir(III)] yielded good linear plots (Fig.3) nearly through the origin. This indicates
that the reaction is of first-order with respect to [Ru(III)].Abtained from
kobs=K[Ru(III)]![]() ( nap=1).
( nap=1).
[HSO4-] was varied in the range of 0.2~1.0 mol·dm-3 at
fixed [H+] ([H+]=1.0 mol·dm-3≈[HClO4]+[H2SO4]), [IBA] and
[Ru(III)]. [HSO4-] was calculated ignoring the dissociation of [HSO4-]
in the presence of fairly high [HClO4]. This leads to [HSO4-]≈[H2SO4]. From table 1, it can be seen that kobs
increases with the increase of [HSO4-]. Therefore HSO4-
shows a rate retarding effect ( nap = 0.20).
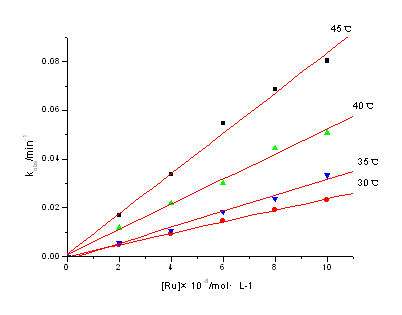
[Ce(IV)]=2.5×10-3 mol·dm-3; [IBA]=0.1mol·dm-3; [H2SO4]=1.0 mol·dm-3; I=1.0 mol·dm-3
Table 1 Effect of varying [HSO4-] on kobs at 298K
[HSO4-]/mol·dm-3 |
[H+]/mol·dm-3 |
103kobs/min-1 |
0.1 |
1.0 |
44.94 |
0.5 |
1.0 |
63.04 |
1.0 |
1.0 |
71.46 |
[Ce(IV)]=2.5×10-3 mol·dm-3, [IBA]=0.1mol·dm-3,·[Ru(III)]=1.0×10-6 mol·dm-3, m=2.0mol·dm-3
3.5 Dependence on [H+]
[H+] was varied over the range 0.2~1.0 mol·dm-3 at fixed [HSO4-]
([HSO4-] = 1.0 mol·dm-3≈[H2SO4]+[NaHSO4]), [IBA] and
[Ru(III)], [H+] was calculated ignoring the dissociation of [HSO4-]
and assuming [H+]≈[H2SO4](Table
2).
Table 2 Effect of varying [H+] on kobs
[H+]/mol·dm-3 |
0.2 |
0.4 |
0.6 |
0.8 |
1.0 |
a |
b |
r |
[ |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.193 |
0.330 |
0.993 |
102kobs/min-1 |
1.989 |
2.368 |
2.731 |
3.130 |
3.329 |
[Ce(IV)]=2.5×10-3mol·dm-3; [Ru(III)]=1×10-7mol·dm-3; [IBA]=0.1mol·dm-3; I=1.0mol·dm-3; t=30
oC; a(intercept), b(slope) and r (correlation coefficient) for the linear regression of kobs vs.H+.3.6 Analysis of product
The completion of the reaction was marked by the complete fading of Ce(IV) color
(yellow). One of the reaction products as isobutyl aldehyde was detected by spot test and
estimated[8], Another product Ce(III) was also detected by spot test[9].
Acrylonitrile solution(40%,V/V) was added to the reaction mixture under
the protection of nitrogen gas, no white deposition could be found, indicating the
reaction system can not initiate polymerization of
acrylonitrile and proving free radicals is not formation in the reaction.
3.7 Mechanism of the reaction
The kinetic data (i.e. 1/kobs vs. 1/[IBA]) fits well with Michaelis-Menten
model[10] , suggesting that 1:1 type complex of IBA and Ru(III) could be formed
in the first preequilibrium step. Ce(SO4)2 has been found
kinetically active in this study, free radicals can be generated in the reaction in acid
media. Thus a mechanism consistent with the found kinetic characteristics is presented as
follows:
![]() (1)
(1)
![]() (2)
(2)
Ru(III)-H![]() Ru(III)+H+ (3)
Ru(III)+H+ (3)
![]() (4)
(4)
[Ru(III)]T =C+[Ru]e
[Ru(III)]e=![]() (5)
(5)
Subscripts T and e means total and equilibrium concentratration
respectively.
From step (2) and (5) the rate of [Ce(IV)]T can be written
as:
![]() =2kobs [Ce(IV)] T =2k[C]=
=2kobs [Ce(IV)] T =2k[C]=![]() [Ce(IV)]T (6)
[Ce(IV)]T (6)
Because of [IBA]>>[Ru(III)],[IBA]T» [IBA]e+[C]» [IBA]e ,
kobs=k[C]=![]() (7)
(7)
![]() (8)
(8)
Eq.(6) indicats that the
reaction is first-
Table 3. Rate Constants and Activation Parameters of the Rate-Determining Step
t / oC |
30 |
35 |
40 |
45 |
activation parameters (30 oC) |
104k/mol-1.L. min-1 |
7.57 |
10.50 |
16.45 |
26.34 |
Ea=54.5 KJ.mol-1, △H≠= 52 KJ.mol-1 △S≠=-149 J.K-1.mol-1, △G≠=97 KJ.mol-1 |
Acknowledgement
This research was supported by National
Science Foundation of China (No. 20675024), Natural Science Foundation of Hebei
Educational Committee (No. 2006407;No.
2008307), China Postdoctoral Science Foundation (No. 2005037629) and Science Foundation of
Hebei University.
REFERENCES
[1] SONG Wen-Yu, LI Wen-Kui, JIA Chun-Ping. Chemical Journal of Chinese Universities,
1999, 20(11): 1767-1771.
[2] SONG Wen-Yu,LIU Hong-Mei. Chinese Journal of
Inorganic Chemistry, 2000, 16(4): 607-611.
[3] Lakshmi S, Renganathan. Int. J. Chem. Kinet., 1996, 28(10):713-720.
[4] SONG Wen-Yu, JIANG Qing-Mei. Acta Chimica Sinica, 2005, 63(2):109-113.
[5] Das A. K., Das M. K. Int. J. Chem. Kinet, 1995, 27(1): 7-16.
[6] Das A. K., DAS M. Indian J. Chem., 1995, 34A: 866-870.
[7] KONG Fan-zhi., TIAN Jian-hua., JIN Zi-lin.. Petrochemical Technology, 2002, 31(5):
387~392.
[8] Feigl F. Spot test in organic analysis. New York: Elsevier pulishing Co., 1956.
P334.
[9] Department of Chemistry, Hangzhou University. Handbook of Analytical Chemistry.
Beijing: Chemical Industry Press, 1982.
[10] Moore J. W., Pearson R. G. Kinetics and Mechanism. New York: John Willey and Sons,
1981. P379.
酸性介质中钌离子催化铈(IV)离子氧化异丁醇的反应动力学及机理
(河北大学化学与环境科学学院,河北省分析科学与技术重点实验室,保定,071002;2、邯郸市外国语学校,邯郸,056000)
摘要 采用氧化还原滴定法,在298-313K温度范围内研究了在酸性介质中钌(III)离子催化铈(IV)离子氧化异丁醇的反应动力学及机理。通过实验发现,反应对钌(III)离子和铈(IV)离子的反应级数都是一级,对异丁醇为正分数级。准一级反应速率常数kobs 随氢离子浓度的增大而增大,随硫酸根离子浓度的增大而减小。在氮气保护下,反应体系不能引发丙烯腈的聚合反应,表明反应过程中没有自由基生成。由此提出了适合此反应的反应机理并求出了相应的活化参数。
关键词 钌(III)离子;铈(IV)离子; 异丁醇; 催化氧化; 动力学及机理