http://www.chemistrymag.org/cji/2009/11a044pe.htm |
Oct.1,
2009 Vol.11 No.10 P.44 Copyright |
Measurement and correlation of liquid-liquid equilibria for quaternary system of water + 2-propanol + dimethyl carbonate + octane
Chen Yao,
Liu Xueen, Xiong Zihao
(Department of Chemistry, Jinan University, Guangzhou, 510632)
Granted by the Ministry of Education of China (No. 200652) and Scientific Research from Guangdong Province of China (No. 2002C32404)
Abstract Experimental tie-line
compositions for one quaternary system of water + 2-propanol + dimethyl carbonate + octane
and one relevant ternary system of water + dimethyl carbonate + octane were measured at
temperature of 298.15 K and ambient pressure. The experimental liquid-liquid equilibria
results were correlated by using a modified UNIQUAC model with ternary and quaternary
interaction parameters in addition to binary ones.
Keywords Fuel additive, Dimethyl carbonate, Liquid-liquid equilibrium, Modified
UNIQUAC model
1. INTRODUCTION
Dimethyl carbonate (DMC) may be an ideal gasoline additive to replace
1,1-dimethylethyl methyl ether (MTBE) because of its excellent characteristics as a
gasoline additive: high octane, low RVP, reduced CO and NOx emissions, and a very high
density [1,2]. DMC has about 3 times the oxygen content of MTBE. It has both
low toxicity and relatively quick biodegradability, and can be considered to be an option
for meeting the oxygenate specification on gasoline and as a means of converting natural
gas to a liquid transportation fuel [3]. To understand the mutual solubility of
DMC with hydrocarbon in gasoline and assess the effect of DMC addition on
hydrocarbon-water mutual solubility, we measured liquid-liquid equilibrium (LLE) data for
quaternary mixtures of water + 2-propanol + DMC + octane, and relevant ternary mixtures of
water + DMC + octane at 298.15 K. The experimental results were correlated by means of the
modified UNIQUAC model [4] including ternary and quaternary parameters, in
addition to binary parameters. The binary vapor-liquid equilibria (VLE), mutual solubility
and ternary LLE relevant to the quaternaries have been available from the literatures:
(2-propanol + octane) [5], (2-propanol + DMC) [6], (2-propanol+
water) [7], (DMC +octane) [8], (Octane +water) [9], (DMC
+water) [10], (water +2-propanol + octane) [11], (water +2-propanol
+ DMC) [12], and (water + DMC + octane) measured in this work.
2. EXPERIMENTS
2.1 Materials
2-Propanol was provided by Tianjin Chemical Reagent Factory with nominal minimum mass
fraction of 0.997. Octane was supplied by the Guangzhou Chemical Reagent Factory with
nominal minimum mass fractions of 0.997. The DMC was obtained from Tianjin Guangfu
Chemical Reagents Factory with nominal minimum mass fraction of 0.992. The gas
chromatograghy analysis gave mass fractions of 0.991 for DMC, 0.997 for 2-propanol and
0.998 for octane. Water was distilled twice and had a mass fraction of 0.999. All
chemicals were used directly in this work.
2.2 Apparatus and Procedures
Ternary and quaternary LLE measurements were carried out at the temperature (298.15 ±
0.01) K. The experimental apparatus and procedures were described in detail previously [13].
The quaternary mixtures whose volume is about 80 cm3 were loaded in the
equilibrium glass cell placed in a thermostated water bath, stirred for 3 h, and then left
to equilibrate for 3 h. The liquid samples about 5 cm3, withdrawn from both
upper and lower phases in the cell by using a milliliter syringe without changing the
phase equilibria between two layers, were analyzed by a gas chromatograph (Shimadzu,
GC-14C) equipped with a thermal conductivity detector. The accuracy of the experimental
measurements was estimated to be within ±0.001.
The quaternary mixtures were prepared by mixing binary mixtures of DMC + octane whose
compositions are M1, M2, and M3 with water then 2-propanol stepwise to cover the two-phase
region shown in Figure 1. The values of M1, M2, and M3 are approximate 0.25, 0.50, and
0.75, respectively, indicating the mole fraction of DMC in the binary mixtures of DMC +
octane. Figure 1 shows schematically a tetrahedron to depict three planes of the
quaternary mixtures of water + 2-propanol + DMC + octane. Experimental LLE data for the
ternary mixtures of water + DMC + octane and quaternary mixtures of water + 2-propanol +
DMC + octane are list in Tables 1 and 2.
Table 1 Equilibrium phase compositions in mole fraction (x) for the ternary
mixtures of water + DMC + octane at 298.15 K
organic phase |
|
||||
x1 |
x2 |
1 – x1 – x2 |
x1 |
x2 |
1 – x1 – x2 |
0.0319 |
0.1181 |
0.8500 |
0.9920 |
0.0080 |
0.0000 |
0.0393 |
0.3586 |
0.6021 |
0.9550 |
0.0450 |
0.0000 |
0.0468 |
0.4836 |
0.4696 |
0.9391 |
0.0609 |
0.0000 |
0.0549 |
0.5505 |
0.3946 |
0.9362 |
0.0638 |
0.0000 |
0.0637 |
0.5627 |
0.3736 |
0.9576 |
0.0424 |
0.0000 |
0.0730 |
0.6108 |
0.3162 |
0.9585 |
0.0415 |
0.0000 |
0.0821 |
0.6609 |
0.2570 |
0.9688 |
0.0312 |
0.0000 |
0.0896 |
0.7540 |
0.1564 |
0.9505 |
0.0495 |
0.0000 |
0.0931 |
0.8086 |
0.0983 |
0.9675 |
0.0325 |
0.0000 |
Table 2 Equilibrium phase compositions in mole fraction x for the quaternary mixtures of water + 2-propanol + DMC + octane at 298.15 K
organic phase |
|
|||||
x1 |
x2 |
x3 |
x1 |
x2 |
x3 |
|
{ x1water + x22-propanol + x3DMC + (1-x1-x2-x3) octane } |
||||||
M1= 0.25 |
||||||
0.0000 |
0.0082 |
0.1240 |
0.8988 |
0.0582 |
0.0398 |
|
0.0000 |
0.0196 |
0.1108 |
0.8623 |
0.0962 |
0.0388 |
|
0.0000 |
0.0188 |
0.1040 |
0.8576 |
0.0993 |
0.0409 |
|
0.0000 |
0.0593 |
0.0858 |
0.8201 |
0.1288 |
0.0466 |
|
0.0000 |
0.0726 |
0.0696 |
0.8090 |
0.1426 |
0.0441 |
|
0.0000 |
0.0854 |
0.0572 |
0.7913 |
0.1543 |
0.0497 |
|
0.0230 |
0.0976 |
0.0575 |
0.7717 |
0.1755 |
0.0468 |
|
0.0281 |
0.1060 |
0.0520 |
0.7491 |
0.1938 |
0.0489 |
|
0.0213 |
0.1184 |
0.0404 |
0.7289 |
0.2050 |
0.0581 |
|
0.0228 |
0.1308 |
0.0330 |
0.6797 |
0.2414 |
0.0671 |
|
M2= 0.50 |
||||||
0.0009 |
0.0412 |
0.2675 |
0.8629 |
0.0836 |
0.0515 |
|
0.0009 |
0.0594 |
0.2107 |
0.8725 |
0.0829 |
0.0431 |
|
0.0139 |
0.0678 |
0.1527 |
0.8241 |
0.1167 |
0.0580 |
|
0.0195 |
0.0773 |
0.1007 |
0.7659 |
0.1795 |
0.0513 |
|
0.0196 |
0.0770 |
0.0877 |
0.6922 |
0.2544 |
0.0497 |
|
0.0196 |
0.0886 |
0.0700 |
0.7286 |
0.2306 |
0.0379 |
|
0.0101 |
0.1149 |
0.0773 |
0.7057 |
0.2257 |
0.0641 |
|
0.0194 |
0.1170 |
0.0650 |
0.6743 |
0.2530 |
0.0665 |
|
0.0169 |
0.1365 |
0.0584 |
0.6613 |
0.2720 |
0.0581 |
|
0.0250 |
0.1469 |
0.0509 |
0.6128 |
0.3034 |
0.0707 |
|
0.0352 |
0.1607 |
0.0900 |
0.5108 |
0.3390 |
0.1204 |
|
M3= 0.75 |
||||||
0.0392 |
0.0653 |
0.5794 |
0.8813 |
0.0570 |
0.0594 |
|
0.0437 |
0.1072 |
0.5946 |
0.8561 |
0.0798 |
0.0626 |
|
0.0411 |
0.1168 |
0.5150 |
0.8473 |
0.0836 |
0.0664 |
|
0.0142 |
0.0741 |
0.2950 |
0.7766 |
0.1118 |
0.1056 |
|
0.0102 |
0.0757 |
0.2052 |
0.7455 |
0.1423 |
0.1082 |
|
0.0131 |
0.0854 |
0.1914 |
0.7294 |
0.1562 |
0.1101 |
|
0.0116 |
0.0852 |
0.1692 |
0.7242 |
0.1630 |
0.1074 |
|
0.0117 |
0.0997 |
0.1596 |
0.6935 |
0.1899 |
0.1098 |
|
3. CALCULATION PROCEDURE AND RESULTS
3.1 Calculation procedure
The modified UNIQUAC [4] model was used to correlate the experimental LLE data. The binary energy parameters,
![]() (1)
(1)
![]() and
and ![]() ( I, II = two liquid phases ) (2)
( I, II = two liquid phases ) (2)
Ternary parameters
t231, t312, and t123 and quaternary parameters t2341, t1342, t1243 and t1234 were determined from the experimental LLE data using a simplex method [15] by minimizing the objective function:F=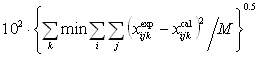 (3)
(3)
where min denotes minimum values, i = 1 to 3 for ternary mixtures or i = 1 to 4 for quaternary mixtures, j = 1, 2 (phases), k = 1, 2, ...,M (number of tie lines), M = 2ni, and x is the liquid-phase mole fraction.
3.2 Calculation results
Table 3 presents the binary energy parameters of the modified UNIQUAC model for the constituent binary mixtures, along with the root mean square deviations between experimental and calculated values: dP for pressure, dT for temperature, dx for liquid-phase mole fraction, and dy for vapor-phase mole fraction. Table 4 shows the ternary parameters obtained in fitting the modified UNIQUAC model to the experimental ternary LLE data, and root-mean-square deviation of the mole fraction of tie lines between the experimental and calculated results. Figure 2 compares the experimental and correlated LLE of the ternary mixtures making up the quaternary mixtures of water + 2-propanol + DMC + octane at 298.15 K. The quaternary system exhibits type 2 quaternary LLE behavior, which is composed of two ternary LLE for the water + 2-propanol + DMC and water + 2-propanol + octane are classified as type 1, and one ternary LLE for the water + DMC + octane as type 2, are illustrated in Figure 2. Table 5 summarizes the correlated results for the quaternary mixtures obtained in fitting the modified UNIQUAC model with binary, ternary, and quaternary parameters to the experimental quaternary LLE data, together with the predicted results by the model with the binary and ternary parameters listed in Tables 3 and 4. The root-mean-square deviation between the experimental results and correlate results is 2.30 %. It seems that the modified UNIQUAC model is able to correlate the quaternary LLE successfully, and the model can give an accurate representation for the quaternary LLE by including the ternary and quaternary parameters in addition to the binary ones.
Table 3 The calculated results of fitting the model to the binary phase equilibria data
System(1+2) |
T/K |
a12/K |
a21/K |
dP/ kPa |
dT/K | 103 dx |
103 dy |
| 2-propanol + octane | 348.15 |
94.46 |
880.92 |
0.3 |
0.1 |
1.2 |
6.6 |
| 2-propanol + DMC | 355.05–361.68 |
230.75 |
66.87 |
0.1 |
0.1 |
1.0 |
8.8 |
| 2-propanol + water | 303.15 |
330.21 |
–44.88 |
0.2 |
0.0 |
1.3 |
8.0 |
| DMC + octane | 361.73–395.56 |
64.24 |
279.30 |
0.2 |
0.1 |
1.2 |
6.8 |
| Octane + water | 298.15 |
3091.80 |
1305.90 |
||||
| DMC + water | 298.15 |
702.87 |
269.81 |
Table 4 The calculated results of fitting the model to the ternary LLE data at 298.15 K
System(1+2+3) |
Na |
Ternary parameters |
Deviationb |
|
| water + 2-propanol + octane | 8 |
t 231 = –0.1043 t132 = 0.2232t123= 0.3844 |
5.80c |
4.10d |
| water + 2-propanol + DMC | 12 |
t231 =
–0.4030 t132 = –0. 2227 t123 = 2.4593 |
6.53 |
0.87 |
| water + DMC + octane | 9 |
t231 = 0.0331 t132 = 0.0117 t123 = 0.0060 | 1.96 |
1.94 |
a
Number of tie-lines.b Root-mean-square deviations (mol %).
c Predicted results using binary parameters alone.
d Correlated results using binary and ternary parameters.
Table 5 The calculated results of fitting the model to the quaternary LLE data at 298.15 K
System(1+2+3+4) |
Na |
Quaternary parameters |
Deviationb |
|
water + 2-propanol + DMC + octane |
|
τ2341 = –1.0287 τ1342 = 1.1297 τ1243 = –61.0614 τ1234 = 42.8804 |
4.34c |
2.30d |
a
Number of tie-lines.b Root-mean-square deviations (mol %).
c Predicted results using binary and ternary parameters.
d Correlated results using binary, ternary and quaternary parameters.
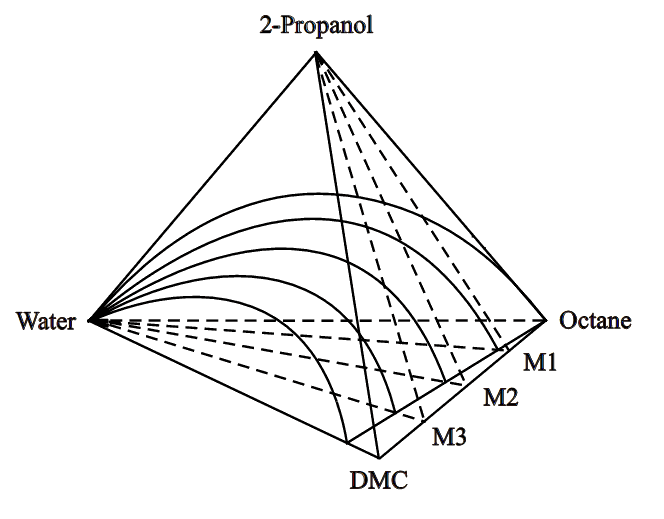
Figure 1 Phase equilibria of (water + 2-propanol + DMC + octane). M1, M2 and M3 denote quaternary section planes
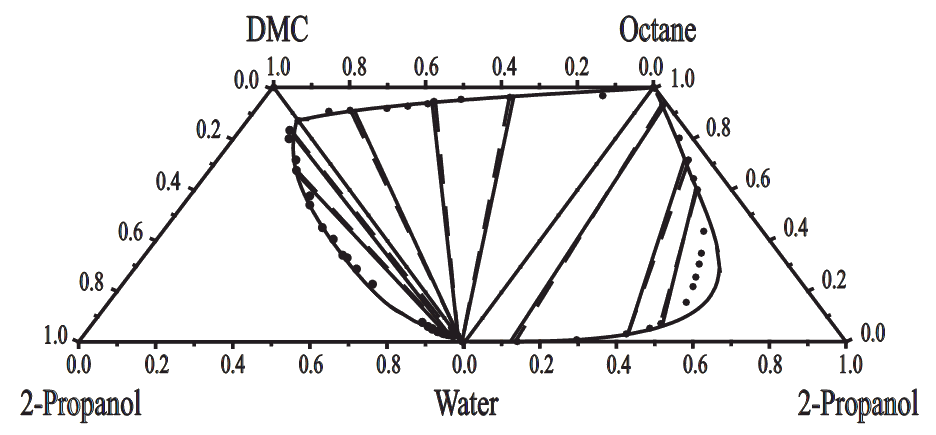
Figure 2 Experimental and calculated LLE of three ternary mixtures making up (water + 2-propanol + DMC + octane) at 298.15 K. ●, experimental tie-line data; ——, correlated curve by the modified UNIQUAC model.
4. CONCLUSION
The experimental tie-line data were measured
for the ternary mixtures of water + DMC + octane and quaternary mixtures of water +
2-propanol + DMC + octane at 298.15 K and atmospheric pressure. The modified UNIQUAC model
is able to represent successfully the experimental liquid-liquid equilibrium results. The
correlated results of ternary and quaternary LLE show a better agreement with the
experimental results.
REFERENCES
[1] Pacheco M A, Marshall C L. Energy Fuels, 1997, 11: 2-29.
[2] Ono Y. Appl Catal A Gen, 1997,155: 133-166.
[3] Fang Y J, Qian J M. J Chem Eng Data, 2005, 50: 340-343.
[4] Tamura K, Chen Y, Tada K, et al. J Solution Chem, 2000, 29: 463-488.
[5] Hiaki T, Tsuji T, Hongo M. Fluid Phase Equilibria, 1996, 125: 79-87.
[6] Pereiro A B, Rodríguez A, Canosa J, et al. J Chem Thermodyn, 2005, 37: 249-257.
[7] Gmehling J, Onken U. Vapor–liquid equilibrium
data collection, Vol. I, Part 1, DECHEMA: Frankfurt / Main, 1977.
[8] Rodríguez A, Canosa J, Domínguez A, et al. Fluid Phase Equilibria, 2002,
198: 95-109.
[9] Sφrensen J M, Arlt W. Liquid-Liquid Equilibrium
Data Collection, Vol. V, Part 1, DECHEMA: Frankfurt, Germany, 1979.
[10] Chen Y, Zhang Y S, Fu M, et al. J Chem Eng Data, 2008, 53(3): 830-837.
[11] Vorobeva A I, Karapetyants M K H. Zh Fiz Khim, 1967, 41: 1984-1989.
[12] Chen Y, Cao C Y. J Chem Eng Data, 2009, 54: 1793-1798.
[13] Chen Y, Dong Y H, Pan Z J. J Chem Thermodyn, 2005, 37: 1138-1143.
[14] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculation for
Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria . Prentice-Hall: Englewood
Cliffs, NJ, 1980:19.
[15] Nelder J A, Mead R. J Computer, 1965, 7: 308-313.
水
+异丙醇+碳酸二甲酯+正辛烷四元体系液液相平衡的测定和关联陈瑶,刘薛恩,熊子豪
(暨南大学化学系,广州,510632)
教育部科学技术研究重点项目教技司(No. 200652),广东省科技计划基金(No. 2002C32404)。
摘要 在298.15K、常压下测定了四元体系水+异丙醇+碳酸二甲酯+正辛烷和一个相关的三元体系水+碳酸二甲酯+正辛烷的液液相平衡数据,并用含有二元、三元和四元相互作用参数的modified UNIQUAC模型对液液相平衡数据进行了关联计算。
关键词 汽油添加剂,碳酸二甲酯,液液相平衡,Modified UNIQUAC模型