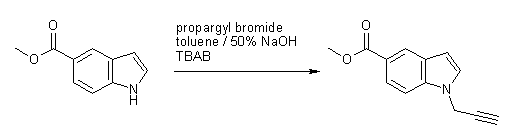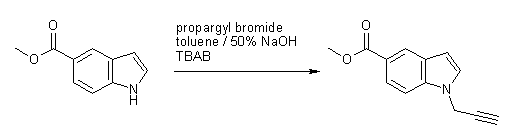Methyl
1-prop-2-yn-1-yl-1H-indole-5-carboxylate
Norbert
Haider * and Tamara Kabicher
Department of Drug and Natural Product
Synthesis,
Faculty of Life Sciences, University of Vienna, Althanstraße 14, A-1090
Vienna, Austria
*
Author to whom correspondence should be addressed. Fax: +43 1 4277
9556; E-mail: [email protected]
Received: 24 August
2007
/ Accepted: 2 October 2007 / Published: 19 November 2007
Keywords:
propargylindole, alkylation, phase-transfer catalysis
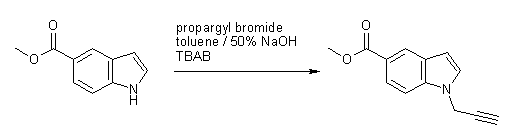
The title compound, which is a
useful synthon in
Sonogashira cross-coupling reactions, can be prepared by N-alkylation
of methyl
indole-5-carboxylate with propargyl bromide in toluene / 50% sodium
hydroxide
under phase-transfer catalysis. Under these conditions [1], the ester
group
remains unaffected and there is no noticeable alkyne/allene
rearrangement [2].
Thus, to a solution of methyl indole-5-carboxylate (1.75 g, 10 mmol)
and
propargyl bromide (2.23 g, 15 mmol of an 80% solution in toluene) in
toluene
(30 mL) was added tetrabutylammonium bromide (0.161 g, 0.5 mmol) and
50%
aqueous NaOH (6 mL). The two-phase system was stirred vigorously for 6
h at
room temperature, then it was diluted with toluene (10 mL) and the
phases were
separated. The organic layer was washed several times with water and
then with
brine. It was dried over Na2SO4
and the solvent was
removed under reduced pressure. The residue was recrystallised from
diisopropyl
ether to give the product (1.432 g, 67%) as yellow crystals.
Melting
point: 94–96 °C
IR (KBr, cm-1): n
3244, 3111, 2947, 2110, 1688, 1613, 1430,
1316, 1200, 1100, 751, 696.
1H-NMR (CDCl3, 300 MHz): d
= 8.41 (d, J4–6
= 1.5 Hz, 1H, 4-H), 7.97 (dd, J6–7
= 8.6 Hz, J4–6 = 1.5 Hz, 1H, 6-H), 7.42 (d, J6-7 = 8.6 Hz, 1H,
7-H), 7.29 (d, J2–3
= 3.3 Hz, 1H, 2-H), 6.64 (d, J2–3
= 3.3 Hz, 1H, 3-H), 4.91 (d, J =
2.6 Hz, 2H, CH2), 3.94 (s, 3H, OCH3),
2.44 (t, J = 2.6 Hz, 1H, C≡CH).
13C-NMR (CDCl3, 75 MHz): d
= 168.02, 138.12, 128.59, 128.36, 124.00,
123.22, 121.94, 108.95, 103.53, 77.09, 73.98, 51.81, 35.93.
MS
(relative intensity): m/z = 213 (M+, 90%), 198
(14), 182 (100), 154
(83), 127 (34), 115 (23), 77 (16), 63 (20), 51 (12).
Elemental
Analysis:
Calculated for C13H11NO2
(213.24): C, 73.23%;
H, 5.20%; N, 6.57%. Found: C, 73.05%; H, 5.20%; N, 6.35%.
References
- Broggini, G.; Bruché,
L.; Zecchi, G.; Pilati, T. 1,3-Dipolar cycloadditions to
nitrogen-substituted allenes. J. Chem. Soc.,
Perkin Trans. 1 1990,
533–539.
- For
recent examples of alkyne/allene rearrangements of N-propargylindoles,
see: a) Haider, N.; Käferböck, J. Intramolecular [4+2] cycloaddition
reactions of indolylalkylpyridazines: synthesis of annulated
carbazoles. Tetrahedron 2004, 60,
6495–6507; b) Abbiati, G.; Canevari, V.; Caimi, S.; Rossi, E. Domino
addition/annulation of delta-alkynylaldehydes and oxygen nucleophiles.
A new entry to [1,4]oxazino[4,3-a]indoles.
Tetrahedron Lett. 2005,
46, 7117–7120.
©
2007 by MDPI (http://www.mdpi.org/).
Reproduction is permitted for noncommercial purposes.