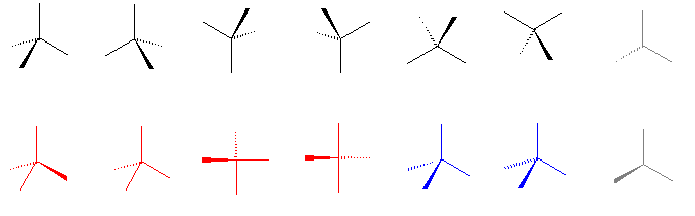
Figure 1. Correct (black and gray) and incorrect (red or blue)
representations of a tetrahedron.
Molecular Diversity Preservation International (MDPI), Saengergasse
25, CH-4054 Basel (New Address: MDPI, Matthaeusstrasse 11, CH-4057
Basel), Switzerland
Tel. +41 79 322 3379, Fax +41 61 302 8918, E-mail: [email protected]
Received: 16 May 1999 / Published: 20 June 1999
Stereochemistry is about the shape and change of shape of molecules. There are several textbooks on this topic. This recent book has a unique feature: Several months after its publication (It was published in October 1998), the author has already established a website [1] for this book at http://www.bangor.ac.uk/ch/mnhome.htm where enhanced diagrams, answers to problems and an updated bibliography are provided and are freely accessible.
This book can be used as reading material for students taking organic chemistry courses and for graduate students. Organic chemists like me can also brush up their knowledge on stereochemistry, particularly organic stereochemistry. The copy I received is a paper-back version. The size and the volume of this book also make reading it comfortable. At the end of each chapter, references for further reading are provided, including a reference to the appropriate chapter of the definative stereochemistry text Stereochemistry of Organic Compounds by Eliel and Wilen [2]
The textbook currently retails at £ 25.00 and can be ordered directly from the publisher by calling +44 1242 267283, faxing +44 1242 253695. You can also order by e-mailing ([email protected]).
I am always interested in how the authors of stereochemistry books use stereochemical representations, because the solid wedge, broken wedge, broken line, solid bar, and broken bar are all frequently used for structural drawing. A large number of combinations of these representations have been seen in literature, which have caused confusion and ambiguity [3]. In this text, stereochemical representation is introduced in Chapter 1. The first figure defines correct (black and gray) and incorrect (red or blue) representations of a tetrahedron (also at the http://www.bangor.ac.uk/ch/mn/booknet/chapter1/c1fig.htm website).

Figure 1. Correct (black and gray) and incorrect (red or blue)
representations of a tetrahedron.
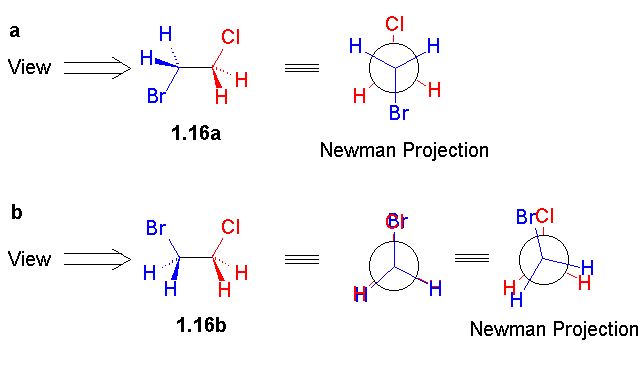
Figure 1.4 of the book, which is reproduced here as Figure 2, is a representative and nice illustration of the graphics contained in this book. Unfortunately the print version has graphics in black and white. Therefore, you may wish to visit this book's website [1], enjoying its colourful figures and 3D molecular structures and further enhanced graphics.
Nomenclature rules for various stereochemical situations are given in chapters 2, 3 and 4.
As mentioned before, there are obvious two aspects (structures and processes, or shape and shape change) to stereochemistry. Chapter 5 is mainly a discussion on stereochemical processes. Chemical analysis and separation of a mixture always depend on the differences in the properties of the components: the more different (distinguishable) the components, the easier they are to separate and analyse. The spontaneity of the opposite process (here racemization, for instance) relies on the components similarity (or indistinguishability): the more similar, more miscible. I have criticized the thermodynamic theory of these two types of process [3]. The tacit belief that the spontaneity of a mixing process is driving by entropy due to the components' difference is highly disputable. Enantiomers are very much the same in almost all properties, except optical rotation. The chemical modification by diastereomerization will enhance differences and separate a racemic mixture. On each occaision that I read a nice text book on stereochemistry, I am impressed by the striking facts of stereochemical processes involving mixing and separation.
The complementarities of enantiomeric compounds with an enzyme or a receptor (original Fig. 3.8, ref.1, p.53) or with a chiral stationary phase (original Fig. 5.4, ref.1, p.107), and between pairs (original Fig. 8.13, p.186) are well presented. In Pauling's classical The Nature of the Chemical Processes, there are only two important keywords, one is resonance, the other is complementarity. The theoretical problems of both resonance (e.g., the rapid degenerate Cope rearrangement in the area of dynamic stereochemistry, which is not covered here due to this book's limited volume) and complementarity are still outstanding.
Finally, this book contributed almost three pages to the very interesting problem of the origin of biomolecular chirality (ref.1, p.54). Physicists call it a symmetry breaking phenomenon. It happened either by chance or not by chance. A recent work by Szabo-Nagy and Keszthelyi [5], if confirmed, would be a great breakthrough in the understanding of the molecular asymmetry evolution of biosphere.
References and Notes
1. North, M. Principles and Applications of Stereochemistry;
Stanley Thornes: Cheltenham, UK, 1998.
Dr. Michael North has a website for this book at http://www.bangor.ac.uk/ch/mn/booknet/bookhome.htm.
2. Eliel, E. L.; Wilen, S. H. Stereochemistry of Organic Compounds; Wiley: London, 1994.
3. (a) Lin, S.-K. A proposal for the representation of the
stereochemistry
of quatrivalent centres. Chirality, 1992, 5,
274-278.
View this paper in html format (http://www.unibas.ch/mdpi/ecsoc/f0007/f0007lin.htm).
(b) Lin, S.-K. Stereochemical representation by wedges and a one-wedge
convention, Invited lecture at "Tetrahedral Carbon's 125th Anniversary
Symposium" (Cosponsored with HIST, Invited Papers Only), Division of
Organic
Chemistry, The 218th ACS national meeting in New Orleans, Louisiana,
August
22-26, 1999 (http://www.mdpi.org/lin/wedge/wedge2.htm).
(c) This convention may be introduced in this journal, the MDPI
published
Molecules
(http://www.mdpi.org/molecules/).
4. Lin, S.-K. The similarity principle (http://www.mdpi.org/lin/similarity/similarity.htm).
5. Szabo-Nagy, A.; Keszthelyi, L. Demonstration of the
parity-violating
energy difference between enantiomers. Proc. Natl. Acad. Sci. USA 1999,
96,
4252-4254 (Reprints are available from Keszthelyi, e-mail:
[email protected]).
Download the text in PDF format. Molecules 1999, 4, 165-168.
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.