http://www.chemistrymag.org/cji/2002/041001pe.htm |
Jan. 1,
2002 Vol.4 No.1 P.1 Copyright |
Microcalorimetric investigation of thermochemical characteristics of the growth metabolism of Tetrahymena pyriformis
Liu Yi1, 2
Sun Dayuan1 Yu Yuhe2 Qu Songsheng1 Shen Yunfen2
(1Department of Chemistry, College of Chemistry and Molecular Sciences, Wuhan
University, Wuhan 430072, China; 2Laboratry of Protozoology, Institute of
Hydrobiology, Chinese Academy of Science, Wuhan 430072, China)
Received Sep.6, 2001; Supported by the National Natural Science Foundation of China (NSFC), and Young Mainstay Teachers' Program of Chinese Educational Ministry.
Abstract The heat output
of the growth metabolism of Tetrahymena pyriformis has been determined by using a
LKB-2277 BioActivity Monitor at 25°C. From the thermogenic curves, it can be established
that thermokinetic equation of their growth metabolism is Pt = Pt=0
exp(km t), dP / dt = km P1
,with the order of growth metabolism n = 1. The experimental results indicate that the
relationship between the metabolic power (P) and the cell concentration (C),
and relationship between the metabolic power of each cell (P0) and the
cell concentration (C) can be characterized by the following thermal equations: P
= A + K C, P0 = A' + K' ln
C or dC / dP0 = C1 .The order of the P0-C equation is also 1. These
results are very significant in environmental sciences, biology and thermochemistry.
Keyword Tetrahymena pyriformis, growth metabolism, microcalorimetry,
thermokinetics, thermal chemical equations.
1. INTRODUCTION
In recent years, biological calorimetry is attracting increasing attention[1].
Microcalorimetry has demonstrated its power as a universal, integral, non-destructive and
highly sensitive tool for many environmental questions, it can provide a lot of kinetic
and thermodynamic information. Several monographs and some articles offer comprehensive
discussion on environmental and biological applications[1-9].
Nevertheless, microcalorimetry can still provide some unexpected surprises and unwanted
pitfalls, they are very significant for study of cell metabolic processes.
Microcalorimetry is very useful for fundamental studies of growth
metabolism of cells. Thus the metabolic process of living cells can be studied through
monitoring the heat effects with sufficiently sensitive calorimeters[10]. In
general, the metabolism of cells is very complicated. In the present work, a LKB-2277
BioActivity monitor has been used to determine the heat output of the growth metabolism of
Tetrahymena pyriformis.
Tetrahymena pyriformis belongs to the protoza family and is
widely distributed, it is an important kind of environmental microorganism. It can be
collected from polluted water, and can be separately cultured into a bacteria-free species
because it is easily cultured and preserved in the laboratory. Tetrahymena pyriformis
is an eukaryotic monocellular animal. It can be used as a biological indicator in
freshwater biology and environment pollution studies. At the same time, it has been used
widely as a "test animal" to
monitor and evaluate toxicants, nutrients, antibiotics, anti-cancer medicaments etc.[11].
2. MATERIAL AND METHODS
2.1 Instrument
A LKB-2277 BioActivity Monitor was used to determine the metabolic power-time curves
of Tetrahymena pyriformis cells. The performance of this instrument and the details
of its construction have been previously described[12-14].
2.2 Materials
Tetrahymena pyriformis (BJ4, mononuclear) was provided by the Department of Biology,
Beijing University.
The culture medium was a solution containing the nutrients peptone 1
wt. %, beef extract 0.1 wt. % and glucose 0.5 wt. %.
2.3 Cleaning procedure for the flow-cell
The flow-cell was cleaned and sterilized as follows: (1) sterilized distilled water
was pumped through the system for 30min at a flow rate of 40ml·h-1; (2) a
0.1mol/L solution of HCl was pumped through the system for 30min at a flow rate of 40ml·h-1;
(3) a 75% alcohol solution was pumped through the system for 30min at a flow rate of
25ml·h-1; (4) a solution of 0.1mol / L NaOH was pumped through the system for
30min at a flow rate of 40ml·h-1; (5) sterilized distilled water was again
pumped through the system for 30min at a flow rate of 40ml·h-1.
2.4 Microcalorimetric determination
Once the system was cleaned and sterilized, sterilized distilled water was pumped
through the system at a flow rate of 10ml / h to run the baseline. After a stable baseline
had been obtained, the bacteria-free Tetrahymena pyriformis species, which had been
cultured pure, was added to 80 ml of liquid medium, and cultured at 25°C by the
cycle-flow method. A schematic representation of the experimental apparatus has been shown
in Fig.1. The preparation was monitored and its thermogenic curves were obtained.
When the pen of the chart recorder starts rising, this indicates that
the Tetrahymena pyriformis cells are entering an exponential growth state. A sample
(3.0 ml) was removed at this stage, 1 ml of 1% formaldehyde solution was added to kill the
organism, and the population density was measured with a haemocytometer.
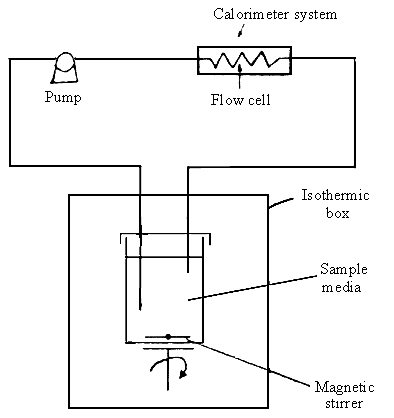
Fig.1 A schematic representation of the
experimental apparatus
3. RESULT AND DISCUSSION
3.1 Growth thermogenic curve of Tetrahymena pyriformis at 25°C
The metabolic processes of Tetrahymena pyriformis in culture media was studied and
the growth thermogenic curve recorded. A typical experimental curve is shown in Figure 2,
there is a turning point ( B ) in the metabolic thermogenic curve, it consists of two
parts, a log phase (AB) and a decline phase (BC). They are two very interesting and
characteristic phase of growth metabolism.

Fig.2 Growth thermogenic curve of Tetrahymena pyriformis
at 25°C
3.2 Thermokinetic equation
In the log phase of growth (AB), the cell is growing exponentially. If the cell number is n0
at time 0, and nt at time t, then
nt = n0 exp(k t) (1)
k is the growth rate constant. If the power output of each cell is w, then
nt w = n0 w exp(k t) (2)
Pt=0 = n0 w and Pt = nt
w, giving
Pt = Pt=0 exp(k t)
or ln Pt = ln Pt=0 + k t (3)
The thermogenic curves of the log phase of growth correspond to Eq.(3).
So, making use of the data Pt and t taken from the curve (shown
as AB part) to fit a linear equation, one can obtain the growth rate constant k.
The rate constant k of Tetrahymena pyriformis growth was shown in Table 1.
Table 1 Rate constants for Tetrahymena pyriformis growth at 28°C
| Experiment No. | 1 | 2 | 3 | 4 | 5 | 6 | mean value |
k (min-1) |
0.0137 | 0.0119 | 0.0122 | 0.0127 | 0.0134 | 0.0132 | 0.0128±0.0006 |
| R | 0.9958 | 0.9965 | 0.9965 | 0.9980 | 0.9997 | 0.9976 | 0.9974 |
From the data in Table 1,
it is apparent that all of the correlation coefficients, R, are greater than 0.9950,
indicating a good reproducibility and correlation-ship.
Data in Table 1 clearly indicate that the
relationship between lnP and metabolic time t satisfies a linear equation,
i.e, for log phase,
ln P = ln P t=0 + km t .
Eq. (3) can be rewritten as
dP /dt = k P = k Pn , n = 1 (4)
Eq. (4) is the thermokinetic equation of Tetrahymena pyriformis growth metabolism,
with the order of growth n=1.
3.3 Data for the growth metabolism
The corresponding P (mW) and C (cells·ml-1) vs. t data of the log
phase (AB) are given in Table 2, and the corresponding P (mW) and C (cells.ml-1)
data of the decline phase (BC) are given in Table 3.
P |
ln P |
C |
ln C |
P0 |
13.7 |
2.617 |
14700 |
9.595 |
0.93 |
*
Volume of the measuring cell is 0.6ml. Table 3 P_t and C--t data for growth metabolism of Tetrahymena pyriformis ( decline phase, 25°C)P |
ln P |
C |
ln C |
P0 |
146.2 |
4.985 |
|
10.624 |
|
3.4 Relationship between P and C
The data of metabolic power (P) and cell concentration (C) in Table 1 and
Table 2 indicate that P and C are linearly related (shown in Fig.3). So, we
can obtain the corresponding linear equations.
For log phase ,
P = -91.4221 + 0.006561 C ,
with correlation coefficient R = 0.9939 (shown in Fig.3 A). For the decline phase,
P = 429.7061 - 0.006733 C ,
with correlation coefficient R = - 0.9906 (shown in Fig.3 B). Particularly, it can be seen
that the P vs. C relationship can be expressed by the common equation
P = A + K C .


Fig.3 Linear relationship of P
vs. C
( A: log phase; B: decline phase )
3.5 Relationship between P0
and C
The values of heat power output by a single cell, P0, are shown in Table
1 and Table 2. The P0 and lnC data taken from Table 1 and Table 2
can also fit linear equations. For log phase,
P0 = -34.5026 + 3.6734 lnC,
with correlation coefficient R = 0.9927 (shown in Fig.4 A). For the decline phase,
P0 = 93.9499 - 8.5039 lnC ,
with correlation coefficient R = - 0.9962 (shown in Fig.4 B). Particularly, that the P0
vs. lnC relationship can be described by the equation
P0 = A' + K' lnC .
This result indicates that power out by a
single cell (P0) depends on the cell population density. In the lag
growth phase, the value of P0 increased with the increasing of cell
concentration (C), it indicates a kind of synergistic action. But, in the decline phase,
the metabolism of cell inhibited by metabolites, the value of P0
decreased with the increasing of cell concentration (C).


Fig.4 Linear
relationship of P0 vs. lnC
( A: log phase; B: decline phase )
3.6 Thermochemical equations
of metabolism
The P-C
data for the growth metabolism were obtained from the thermogenic curve in Figure 2. As
described above, P and C are linearly proportional (see Figure 3). P
and C relationship is written as:
P = A + K C .
Using the P0 and lnC values from
Tables 1 and 2 to fit a linear equation (see Figure 4), the relationship between P0 and lnC should be
P0 = A' +K'
lnC.
Hence, we can obtain
dC / dP0= K' Cn , n = 1.
with the order of metabolism n = 1 . So, the thermochemical equations of growth metabolism
was obtained.
3.7 The mean heat power
output of each cell
From the P0 data in Table 1 and Table 2, we can calculate the
mean heat power output of each cell at different phase. In the log phase, P0
is 2.32±1.06 nW.cell-1. P0 is
1.86±1.16 nW.cell-1 in the decline phase. These values
are close to the value in reference [15].
4. CONCLUSION
The growth metabolism of Tetrahymena pyriformis cells has been determined. The
experimental results indicate that the relationship between cell concentration and heat
output can be characterized by the equations,
P = A + K C , P0 = A' + K' lnC
or P = A + K C, dC / dP0 = K' Cn
for Tetrahymena pyriformis growth metabolism n = 1.
The growth thermokinetic equation is
Pt = Pt=0 exp( km
t) or d P / d t = km P1
with the order of growth n = 1.
In all of these equations, km,
n, K, A, K' and A' are constants for metabolism. These equations are characteristic
equations for the growth metabolism of Tetrahymena pyriformis . These equations
indicate that the metabolic power linearly correlated to the cell concentration, and that P0
(the single cell metabolic power output) depends on the cell concentration.
The experimental results confirmed the applicability of the equations,
with good linear correlationship. In general, the growth metabolism of Tetrahymena
pyriformis cells can be described by these equations. These equations specifically
characterized the growth metabolic process of the Tetrahymena pyriformis and
provided a functional relationship for the growth metabolism of environmental protozoa.
All of these results are significant of environmental sciences.
REFERENCES
[1] Holzel R, Motzkus C, Lamprechet I. Thermochimica Acta, 1994, 239: 17.
[2] Mcgulnness S M, Barisas B G. Environ. Sci. Technol., 1991, 25: 1092.
[3] Beezer A E. Biological Microcalorimetry. London: Academic Press, 1980.
[4] James A M. Thermal and energetic Studies of cellular Biological Systems. Wright
Bristol, 1987.
[5] Wadso I. Thermochimica Acta, 1993, 219: 1.
[6] Kemp R B, Schon A. Thermochimica Acta, 1990, 172, Special issue.
[7] Lamprecht I, Hemminger W, Hohne G. Thermochimica Acta, 1991, 193, Special issue.
[8] Kemp B, Schaarschmidt B. Thermochimica Acta, 1995, 250 (2): Special issue.
[9] Kemp B, Schaarschmidt B. Thermochimica Acta, 1995, 251: Special issue.
[10] Xie C L, Sun D Y, Song Z H et al. Thermichimica Acta, 1989, 142: 211.
[11] Zhang Z X, Proc. 4th Symp. of the Chinese Protozological Society, China, Nov.10-14,
1987.
[12] Xie C L, Tang H K, Qu S S et al. Thermichimica Acta, 1988, 123: 33.
[13] Suurkuusk J, Wadso I. Chem. Scr., 1982, 20: 155.
[14] Yan C N, Liu Y, Qu S S et al. Chemosphere, 1999, 38 (4): 891.
[15] LKB-2277 Bioactivity Monitor Literature Reference List.