http://www.chemistrymag.org/cji/2002/042010pe.htm |
Feb. 1,
2002 Vol.4 No.2 P.10 Copyright |
Lu Jianmei, Dai Weiquan, Zhu Xiulin, Wang Lihua, Zhang Zhengbiao
(Chemistry Department of Suzhou University, China, 215006)
Received Dec. 30, 2001; Supported by National Natural Science Foundation of China (No. 20076031) and Natural Science Foundation of Jiangsu province (No. BK99081)
Abstract Solid phase coordination reaction of europium ion (III)(Eu3+) and the resultant of imidization of polycondensor of Benzoguanamine (BGA) and 2,4-tolylenediisocyanate (TDI) polycondensation and pyromellitic dianhydride (PMDA) under microwave radiation were synthesized and studied. The effects of microwave radiation time (power), the composition of two reactants and the reaction temperature on the yield and Eu content in the complexes were studied. The complexes were determined by Fourier transform Infrared absorption (FTIR), Fourier transform Raman spectrum (FTRS), 13C solid state nuclear magnetic resonance spectrometry, scanning electric minor (SEM) and X-ray powder diffraction, and the Eu content in the complexes were measured. The possible structure of complex was inferred. The fluorescence intensity was measured by fluorescent emission spectrum and the same intensity was compared with that from thermal coordination. The results showed that the complex prepared can emit fairly strong characteristic fluorescence of Eu3+ and the temperature of coordination saturation was lower than that of thermal coordination. The experimental results also showed that the fluorescence intensity increased with increasing Eu content, which illustrated that Eu3+ dispersed uniformly in the complexes.
Keywords Microwave radiation; Solid phase coordination; Polyimide-rare earth metal complexes; Fluorescence properties
1 INTRODUCTION
Solid phase coordination chemistry is a frontier science between solid phase chemistry and coordination chemistry, which studied solid phase reaction of coordination compounds at low temperature (room temperature-200ºC) .Microwave was first applied in organic chemical synthesis by Gedye in the 1980s, which was brought a deal of attention and the range of research has been larger and larger, so an intersect science ----microwave chemistry was taken shape. Microwave has many advantages, such as heating inside, heating quickly, heating selectively, saving energy and so on. At present, microwave radiation has been extensively used in inorganic chemistry and organic chemistry [1-4], but using in polymerization reaction only just began [5]. The study of solid phase coordination reaction at low temperature was very few, and the coordination reaction of polymers under microwave radiation has not been reported. Recently in our laboratory, polyimide-rare earth metal complexes, and their fluorescent properties and magnetic properties were investigated.
2 EXPERIMENTAL
2.1 Instrument
Domestic multimode 800W microwave oven , modified domestic microwave oven
2.2 Material
2.2.1 BGA-TDI-PMDA Polyimide
Benzoguanamine was dissolved in anhydrous DMF, nitrogen gas was introduced to the solution. TDI and triethylenediamine were added into the solution. Stirred to dissolving perfectly, PMDA was added to the solution for three times. The temperature of polymerization was controlled by modulating the power of the microwave oven. After 1h , the solution was poured into methanol, and the precipitate was washed with THF and dried under vacuum at 45ºC overnight . PU and PMDA were mixed and rubbed, and the mixture was dehydrated by microwave radiation for 10 minutes. The product was washed with THF and dried under vacuum at low temperature.
2.2.2 Europium chloride (EuCl3·nH2O)
Europium oxide was dissolved in dilute hydrochloric acid (6mol/L), and the solution was heated at 120ºC.The chloride product formed was white crystal(melting point: 149.5ºC - 150.5ºC).
2.2.3 Preparation of complex (Eu3+-BGA-TDI-PMDA polyimide) by microwave radiation
The polyimide and EuCl3onH2O was mixed with a given proportion and rubbed perfectly .The mixture was put into a domestic microwave oven and reacted under continuous and intermittent radiation for some time at a given temperature. The product was washed with anhydrous ethanol and DMF in turn and ether for several times. Then the complex was dried under vacuum and weighed.
2.2.4 Preparation of complex (Eu3+-BGA-TDI-PMDA polyimide) by conventional heating method
The mixture as which above-mentioned was put into the oven which temperature has been set up (the same temperature as the maximum temperature of the microwave radiation), and reacted for some time. Then the mixture was dealt with the method as above.
2.2.5 Determination of Eu content in complex
The amounts of Eu in the complex were determined by a method of EDTA titration [6].
2.2.6 Spectral Measurements
IR spectra of the polymer and the complexes were measured with a Mangna-550. FR spectra of the polymer and the complexes were measured with a Nicolet FT-Roman 960. SEM of the complexes was measured with S-570. 13C solid-state nuclear magnetic resonance spectrometry was measured with Bruker ARX-300. X-ray microanalyses were measured with a DMAX-3C.
A SPECTRAPRO 750i fluorescence spectrophotometer was used to measure fluorescence emission spectra of complexes. Powder samples were used for the measurements. lex=532nm.
3 RESULTS AND DISCUSSION
The complexes were not dissolved in the solvents.
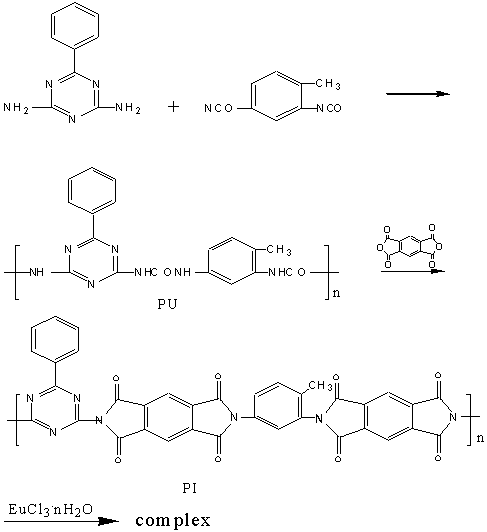
The coordination structure of Eu3+-polyimide complex was investigated by measuring the IR spectra(Fig.1,2). The polyimide has absorption bands around 1780cm-1 and 1720cm-1 corresponding to C=O (=N-C=O), and 1590cm-1 corresponding to C=N, and 1361cm-1 corresponding to C-N. But these bands in complexes shift to 1727cm-1, 1654cm-1, 1554cm-1 and 1384cm-1 respectively. These shifts suggest complexation between Eu ion and N or O atom [7,8].
The coordination structure of Eu3+-polyimide complex was also investigated by measuring the RR spectra (Fig.3, 4). The polyimide has absorption bands around 1603cm-1, 1392cm-1 corresponding to C=N vibrating absorption band, nitrogen heterocycle stretching vibration. The bands in the complexes shift to 1613cm-1, 1433cm-1 respectively. On the other hand, the polyimide has absorption bands around 1720 cm-1 and 1684cm-1 corresponding C=O stretching vibration. The bands in complexes become very feeble. These shifts also suggest complexation between Eu ion and N or O atom [9, 10].
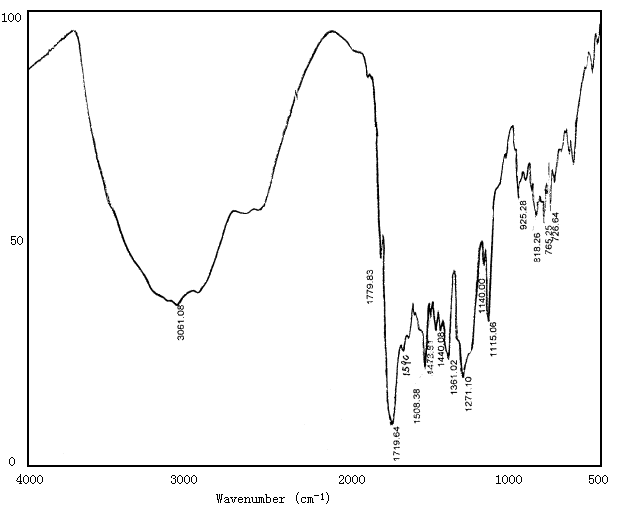
Fig.1 IR of polyimide
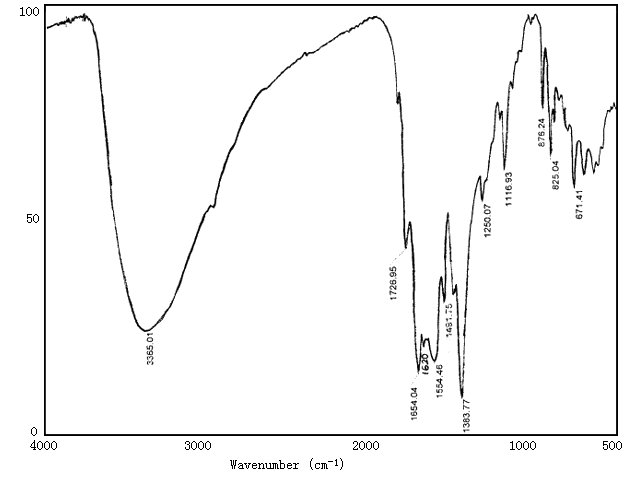
Fig.2 IR of Eu3+-polyimide

Fig.3 FTRS of polyimide
Fig.4 FTRS of Eu3+-polyimide
In 13C solid state nuclear magnetic resonance spectra of polyimide(Fig.5), the peak at 175ppm, 170ppm and 165ppm corresponding to C=O. But in spectra of complex (Fig.6) the peaks shift to 166ppm, 156ppm and 130ppm respectively [11,12].
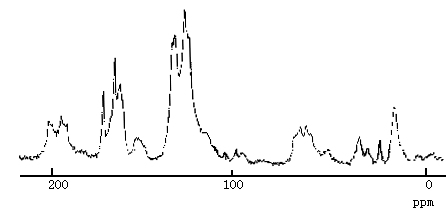
Fig.5 13C spectra of polyimide
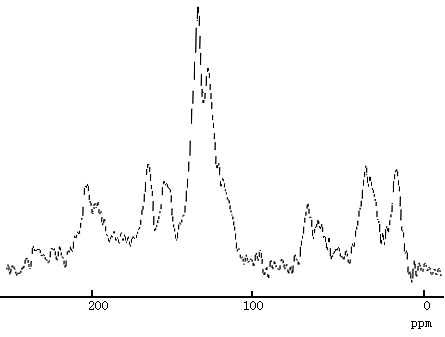
Fig.6 13C spectra of Eu3+-polyimide
X-ray diffraction patterns of EuCl3·nH2O(Fig.7), polyimide(Fig.8), the mixture of EuCl3·nH2O and polyimide(Fig.9) and the complex(Fig.10) are different, which suggests that complex is a new matter, not the simple mixture of EuCl3·nH2O and polyimide.
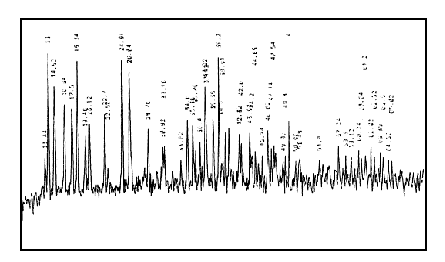
Fig.7 X-ray diffraction patterns of EuCl3·nH2O

Fig.8 X-ray diffraction patterns of polyimide
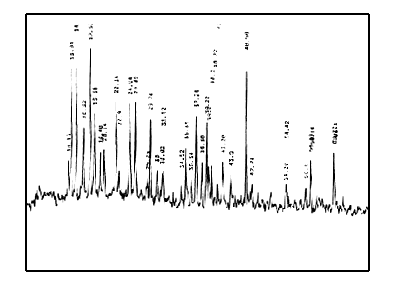
Fig.9 X-ray diffraction patterns of mixture of EuCl3·nH2O and polyimide
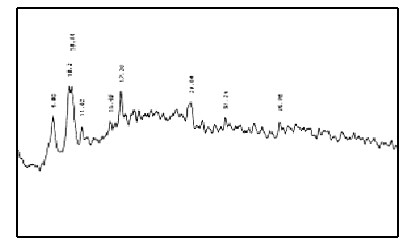
Fig.10 X-ray diffraction patterns of the complex
The result of the SEM of the complex showed in Fig.11. According to the compution of the ZAF revised method [13], The proportion of atom number of Eu and Cl was 1:1. Because the complexes and the solvent used have no charge, but Eu ion has three positive charges, so 2Cl- may be replaced by 2OH- of the H2O.
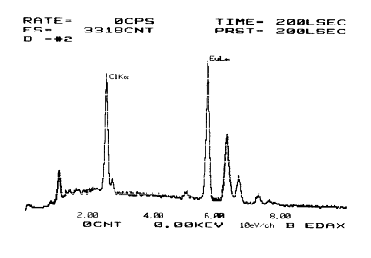
Fig.11 SEM of the complex

Fig.12 Emission spectra of polyimide
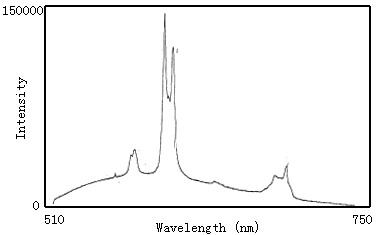
Fig.13 Emission spectra of Eu3+-polyimide
In the emission spectra of polyimide and complex, the polyimide has a wide peak around 600nm(Fig.12). But the complex behaves fluorescence properties of the Eu3+. In spectra of complex(Fig.13), the peaks at 580nm, 590-596nm, 610-620nm, 650nm and 687-703nm are due to the 5D0¡ú7F0£¬5D0¡ú7F1£¬5D0¡ú7F2£¬5D0¡ú7F3 and 5D0¡ú7F4 transition. The peak at 613nm due to 5D0¡ú7F2 is the most strong, and the followed fluorescence intensity of this article is the intensity of peak at 613nm.
3.1 Possible structures of the complex
From the results of SEM, one Eu atom must coordinate with 1Cl- and 2OH -. Because the complex is not dissolved in any solvent, there must have netlike structure in the complex, and the number of coordination is 5 at least. According to results of the spectra, there are complexation between Eu ion and N or O atom. If one Eu ion coordinates with two atoms (N or O) of two polymer chains, the coordination number is 5. If one Eu ion besides coordinates with one N atom and one O atom of a structural unit of a polymer chain, it also coordinates with N atom or O atom of other unit or other polymer chain, the coordination number may be 6, 7 or 8.
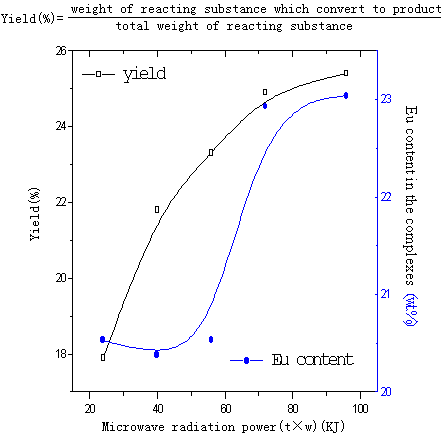
Fig.14 influence of microwave radiation power on complexes
(Eu ion vs polymer structure units is 4mmol/1mmol, at 90ºC)
3.2 Influence of microwave radiation
power(t¡Áw) on complexes
In Fig.14, yield of complex and content of Eu ion in the complexes were
found to increase with increasing microwave radiation power. The possible reason is that
solid phase reaction includes four steps (diffusion, reaction, nucleation and growing)[14].
When the radiation energy added, the time of Eu ion and polyimide reacting is longer. On
the other hand microwaves can cause rapid shift of the dipoles in the molecules, this
process which may be viewed as molecular agitation or stirred could replace mechanical
stirring[15], so the reaction group of the polyimide enwrapped in the tangled
polymer chains exposed and reacted with Eu ion or shorten distance between Eu ion and
reaction group which is far originally to have chance to react. In Fig.10, the
fluorescence intensity increased with increasing Eu content in the complex, which suggests
that Eu ion disperse uniformly in the complex [16].
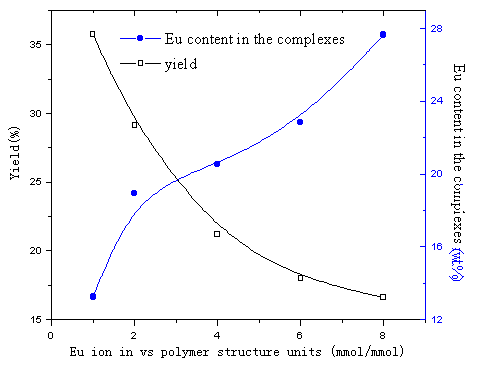
Fig.15 influence of composition of reactants on Eu content in complexes and
yields of the complexes
(time of reaction is 70min, at 90ºC)
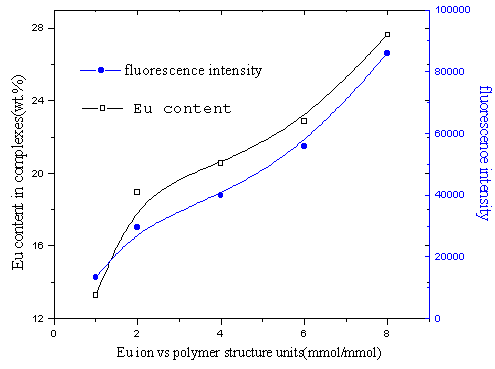
Fig.16 influence of composition of reactants on Eu content in complexes and
fluorescence intensity
(time of reaction is 70min, at 90ºC)
3.3 Influence of proportion of Eu ion and polyimide on the complexes
In Fig.15, Eu content in the complexes increased with increasing Eu
content in the reactants. However, the yields of the complexes decreased with increasing
Eu content in the reactants. The possible reason is that at the beginning of the reaction,
the more quantity of Eu ion added in the reactants, the more ion coordination with the
polymer, intermolecular complex was mainly formed. Later, intramolecular complex formed
mainly and increased quantity of Eu ion in the complex was less than increased quantity of
the reactants, which leads to decreasing yields of the complexes. In Fig.16, in the
complexes the fluorescence intensity increased with increasing Eu content, which
illustrate that Eu ion disperse uniformly in the complexes[16].
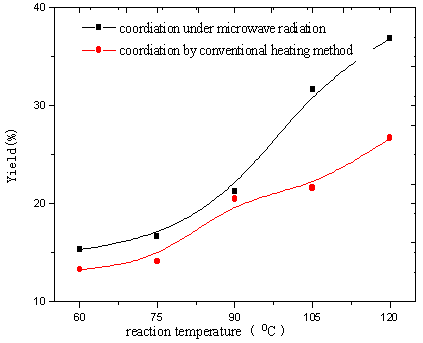
Fig.17 influence of reaction temperature on
yield of complexes
(Eu ion vs polymer structure units is 4mmol/4mmol, for 70min)

Fig.18 influence of reaction temperature on
Eu content in complexes
(Eu ion vs polymer structure units is 4mmol/1mmol, for 70min)
3.4 Coordination by microwave
radiation compared with conventional heating method
From Fig.17 and 18, the yields of the complexes and Eu content of the
complexes were found to increase with the increasing of reaction temperature of two
methods. It was illustrated that higher temperature promoted reaction. Because diffusion
is the slowest step of phase reaction and reaction rate is decided by diffusion rate.
Raising reaction temperature is in favor of diffusion [13]. At higher
temperature especially higher than 100ºC, volatilized crystal water of EuCl3onH2O
formed liquid bridge between Eu ion and polymer and lacuna of crystal lattice formed when
H2O and HCl left reaction system, which were in favor of reaction. In the complexes the
fluorescence intensity increased with increasing Eu content (Fig.19, 20). It was suggested
that Eu ion was dispersed uniformly in the complex. Fluorescence intensity of complexes
under microwave radiation was stronger than that of complexes using conventional heating
method at the same reaction temperature (Fig.21). Because transport properties and
diffusion properties of molecules improved by microwave radiation, which enhanced reaction
rate. So microwave radiation may have both thermal activation and non-thermal interaction
[15].

Fig.19 influence of reaction temperature on comlpexes under microwave radiation
(Eu ion vs polymer structure units is 4mmol/1mmol, for 70min)

Fig.20 influence of reaction temperature on
complexes by conventional heating method
(Eu ion vs polymer structure units is 4mmol/1mmol, for 70min)
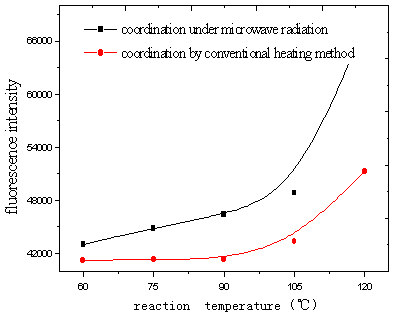
Fig.21 influence of reaction temperature on
fluorescence intensity by two methods
(Eu ion vs polymer structure units is 4mmol/1mmol, for 70min)
REFERENCES
[1] Gedye R, Smith F, Westaway K. Tetrahedron Lett., 1986, 27 (3): 279.
[2] Rama Rao A V, Gurjar M K, Kaiwar V. Tetrahedron Asymm., 1992, 3: 859.
[3] Puciova M, Ertl P, Toma S, Collect. Czech.Chem.Commun., 1994, 59: 175.
[4] Baghurst D R, J. Chem. Soc. Commun., 1988, 11: 829.
[5] Silinski, Brigitte, Kuzmycz, Clande. Eur. Polym. J., 1987, 23 (4): 273.
[6] Jiang Z C, Cai R X, Zhang H S. Rare Earth Elements Analytical Chemisty in Chinese
(Xitu Yuansu Fenxi Huaxue), Science Publish (Keji Chubanshe), 3rd, 2000
[7] Iftikhak, Sageed M, Ahmad N. Bull Chem. Soc. Japn., 1982, 55: 2258.
[8] Zhao Z P, Zhou X R. Apparatus Analytical in chinese (Yiqi Fenxi), Superior education
Publish (Gaodeng Jiaoyu Chubanshe), 1995.
[9] Meconnell A A, Brown D H, Smith W E. Spectrochim.Acta, 1982, 38A (2): 265.
[10] Zhu Z Y, Gu R A, Lu T H. Roman Spectrum Applied in Chemisty in chinese (Laman Guangpu
Zai Huaxue Zhong De Yingyong), Northeast University Publish (Dongbei Daxue Chubanshe),
1998.
[11] Britemell E, Walter W, translated by Liu L X and Tian Y Z, 13C Solid NMR
Spectrum in chinese (Guti Heci Gongzheng Bopuxue), Dalian industry Publish (Dalian Gongye
Chubanshe), 1986.
[12] Tan C Q, Hu J H. J. of Spectroscopy in chinese(Bopuxue Zazhi), 1998, 15 (4): 379-382.
[13] Goldstein J I. Scanning electron microtechnique and x-ray microanalysis, 1988, 34-80;
137-250.
[14] Miu Q, Xin X Q, Hu C. Chinese Journal of Chemical Physics (Wuli Huaxue Xuebao), 1994,
7 (2): 118.
[15] Jacob J, Chia L H L. Journal of Materials Science, 1995, 30:5321-5327.
[16] Banks E, OkamotoY, Ueba Y. J. Appl. Polym. Sci., 1980, 125: 359-368.
¡¡