http://www.chemistrymag.org/cji/2002/046028ne.htm |
Apr. 6, 2002 Vol.4 No.6 P.28 Copyright |
Wang Jingping, Zhou Jing, Du Liming
(Analytical and Testing Center, Shanxi Normal University, Linfen Shanxi, 041004)
Recieived Feb. 27, 2002; The Natural Science Foundation of Shanxi Province (No. 20011016)
Abstract The fluorescence intensity of
fleroxacin (FLX) can be enhanced greatly in sodium dodecyl sulfate (SDS) micellar system.
This finding provides a new method for potential application in the FLX trace
determination. The linear range of concentration is 0.07-0.79 mg/mL and the detection limit is 0.07 mg/mL, the average recovery is
within the range of 99.4-101.6%, and the relative standard deviation is 1.3-2.0 %.
Keywords Fleroxacin, Sodium dodecyl sulfate, Fluorescence
1 INTRODUCTION
Fleroxacin (FLX) is the new generation antibiotics of the quinolone family. Due to its
intensive and efficient antibacterial activity, and the features of better bioavailability
as well as lower side effect, it has been widely used in clinical practice[1].
The related analytical methods reported are HPLC[2-4] and capillary zone
electrophoresis[5,6] et al.. On the basis of our previous results of the FLX
fluorescence properties [7], we have carried out investigations into the FLX
fluorescence sensitivity in micellar systems. It was found that the fluorescence intensity
is enhanced considerably with sodium dodecyl sulfate (SDS) in slight acid medium. This
result provides a new possibility for the determination of trace FLX. This method is easy
for use, and has high sensitivity as well as good selectivity, thus provides satisfactory
results.
2 EXPERIMENTAL
2.1 Apparatus
All fluorescence spectra were recorded on a Perkin Elmer LS-50B spectrophotometer with a 5
nm band pass for excitation and emission. The acidity of the system was determined on a
PHS-3 acidometer (Shanghai, Tianda, Instrument Co. Ltd)
2.2 Reagent
Fleroxacin reference sample (99.5%), offered by the Institute of Pharmaceutical Product
Control of China, was dried at 105 oC. A 5¡Á10-3 mol/L stock FLX
solution was prepared with twice distilled water by adding several drops of 0.1 mol/L NaOH
solution, and further diluted to the final volume and concentration as needed. A 0.1 mol/L
sodium dodecyl sulfate (SDS) solution was also prepared. Buffer solutions with different
pH ranges were prepared by mixing a 0.04 mol/L B(OH)3/H3PO4/CH3CO2H
stock solution and a 0.2 mol/L NaOH solution. All reagents have analytical purity and
water was twice distilled in a sub-boiling distiller.
2.3 Procedures
A given volume of the prepared FLX and 2.5 mL SDS solutions were in turn piped into a 25
mL comparison tube, which was filled with the prepared pH = 3.8 buffer solution to the
scale. The final solution was shaken and mixed well for the fluorescence measurements with
the excitation and emission wavelengths at 286 nm and 445 nm respectively. The blank
experiments were also done at the same time.
3 RESULTS AND DISCUSSION
3.1 Excitation and emission spectra
As shown in Figure 1, FLX can emit fluorescence with low intensity. However, adding
appropriate amount of the SDS into the FLX solution can enhance the fluorescence intensity
greatly. This is due to the formation of the SDS micelle, which reduces the intramolecular
deactivation process in the microenvironment and the fluorescence quenching effect. For
measurement, we selected the excitation and emission wavelengths at 286 nm and 445 nm
which show the large enhancement.
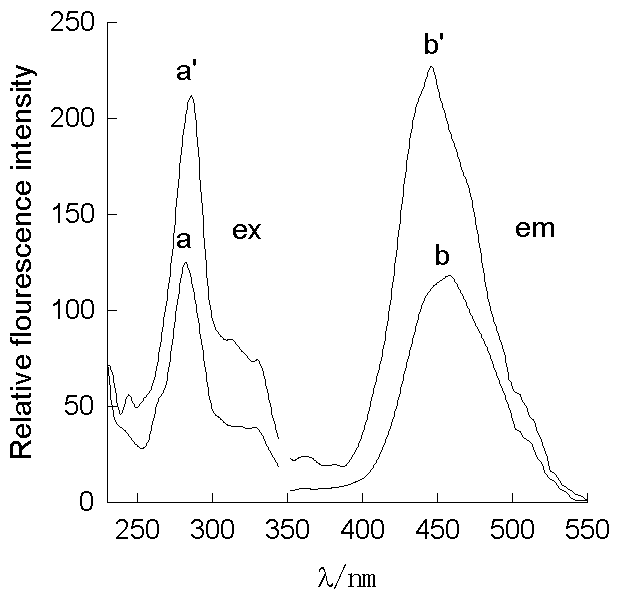
Fig.1 Excitation and emission
fluorescence spectra
a, b FLX lex/ lem= 286 nm /450 nm;
a' b' FLX / SDS lex/
lem= 286 nm /445
nm;
[FLX] = 7¡Á10-7 mol/L; [SDS]=8¡Á10-3 mol/L; pH =3.8
3.2 Effect of pH
Since FLX has 3-carboxyl-4-oxygen quinolone functional group, as well as nitrogen and
oxygen atoms, the degree of protonation in different acid or basic solutions will affect
the electronic properties of the emission systems, and the acidity and basicity will
change the FLX/SDS fluorescence intensity considerably.At 286 nm and 445 nm wavelengths,
the relationship of the fluorescence intensity with acidity (pH, controlled by buffer
solution) was investigated. It was found that the FLX/SDS fluorescence intensity increased
with the acidity decreasing, and reached the maximum at pH = 3.8. Along when pH > 4,
the intensity decreased. Therefore, pH control during the measurement is highly necessary,
pH = 3.8 should be set in this measurement.
3.3 Effect of surfactants
After studying the influence of various surfactants on the FLX fluorescence intensity, it
was found that non-ionic surfactants Brij-35, Tween-20, Tween-80 and Triton X-100 did not
have any strong enhancement effect, and cationic surfactants (CTAB and CPDB) have some
effects. However, anionic surfactants (SDS and SDBS) have very great enhancement effects,
and SDS performs the best. At the same time, we found that the micelle concentration has
large effect on the fluorescence intensity. The intensity reaches the maximum on the
concentration of 6.0¡Á10-3 ~ 1.0¡Á10-2 mol/L, and decreases rapidly
as that larger than 1.0¡Á10-2 mol/L. Thus the SDS concentration of 8.0¡Á10-3
mol/L is used as the best condition in this work.
3.4 Standard curve
Under the optimized condition, we found the good relationship between the FLX
concentration and fluorescence intensity within the range of 0.07-0.79 m g/mL (F = 833.33CFLX
+ 50, r = 0.9960). With the ratio of signal to noise S/N = 3, the detection limit is 0.07m
g/mL.
3.5 Results of sample detection
The same procedure described above was used for FLX tablets solution in a given
concentration for five parallel measurements. The results were fitted into the linear
relationship between concentration and intensity for calculating the FLX concentration and
conducting the standard recovery experiment. The recovery results are listed in Table 1.
Table 1 The recovery for FLX tablets
NO. |
Lot number |
Original
amount |
Amount added |
Amount found (mg/mL) |
Average
recovery |
RSD |
1 |
010400380 |
0.05 |
0.11 |
0.158,0.162,0.155,0.163,0.164 |
101.6 |
2.0 |
2 |
010400440 |
0.05 |
0.22 |
0.271,.0273,0.268,0.265,.0.266 |
97.0 |
1.3 |
1,2 Fleroxacin tablets£¨Products of Henan Topefond Pharmaceutical Co LTD £©
REFERENCES[1] Weidekamm E. J Antimicrob Chemother, 1992, 36: 1909.
[2] Jiao Guohua, Li Baoyou, Zhang Qichang. Chinese Journal of Pharmaceuticalis (Zhongguo Yaoxue Zazhi) ,1996, 27 (11): 519-520.
[3] Eva M G, Alfredo C, Alddreas H, Thomas A. Ternas. Anal. Chem., 2001, 73 (15): 3632.
[4] Heizmann P, Dell D, Eggers H. J. Chromatogr., Biomed. Appl., 1990, 92 (1): 91.
[5] Niu yujuan, Zhang Shixuan. J. Pharmaceutical Anal (Yaowu Fenxi Zazhi), 2001, 21 (3): 204.
[6] Zhang Ruiping,Wang Shufang, Pan Jinghao. China J Antibioties (Zhongguo Kangshengsu Zazhi), 1998, 12 (6): 431-432.
[7] Du Liming, Jin Weijun, Dong Chuan et al. Spetroscopy and Spectral Analysis, 2001, 21 (4): 518.
¡¡