http://www.chemistrymag.org/cji/2002/046027pe.htm |
Apr. 6, 2002 Vol.4 No.6 P.27 Copyright |
Graft copolymerization of acrylonitrile onto chitosan initiated by potassium diperiodatonickelate (IV)
Liu Yinghai, Liu Zhenghao, Zhang Yanzhe, Deng Kuilin(College of Chemistry & Environmental Science, Hebei University, Baoding 071002, China) Received Dec. 30, 2001; Supported by Natural Science Foundation of Hebei Province
Abstracts A novel redox system, potassium diperiodatonickelate [Ni (IV)]- chitosan, was employed to initiate the graft copolymerization of acrylonitrile (AN) onto chitosan. The effects of such reaction variables as monomer concentration, initiator concentration and temperature on the graft copolymerization were investigated. Ni (IV)- chitosan system is found to be an efficient redox initiator for this graft copolymerization. A two-step single electron transfer mechanism is proposed to explain the formation of radicals and the initiation. The grafted copolymers were identified by Fourier transfer infrared spectroscopy (IR) and X- ray diffraction diagrams. The thermal stability of chitosan and chitosan- g- PAN was studied by thermogravimetric analysis (TGA).
1 INTRODUCTION
The basic theoretical research and application on supernormal valence transition- metal
attract more and more attention in chemical fields nowadays. In resent years, some
achievements have been obtained on vinyl monomer polymerization and graft copolymerization
initiated by supernormal valence transition- metal, such as Mn (VII), Cr (VI), V (V), Ag
(III), Cu (III) [ 1-4] etc. But there are few reports on graft copolymerization
of vinyl monomer onto chitosan using potassium diperiodatonickelate [Ni (IV)] redox system
as initiator. Normally, it is believed that the mechanism of oxidation by Ni (IV) is a
two- electron- transfer process without radicals, and Ni (IV) cannot initiate
polymerization of vinyl monomer [ 5-9] . However, under our experimental
conditions, the results show that radical polymerization of some monomers can be initiated
by Ni (IV) redox system [ 10- 11] . So, the two- step single electron transfer
mechanism is proposed to explain the generation of radicals and the initiation.
Chitosan, obtained from chitin poly- b - (1, 4)- N-acetyl- D-
glucosamine through deacetylation using strong aqueous alkali solution, is a more
versatile form of this polysaccharide, which is the second most abundant natural polymer
on earth after cellulose. Grafting various monomers onto its backbone is a promising
method for the preparation of new materials, which have the potential and multiple
applications due to their improved chemical and physical properties. Many grafting
copolymers of chitosan and vinyl monomers were synthesized and evaluated as flocculant,
paper strengthener, drug-releaser and so on [12-17].
2 EXPERIMENTAL
2.1 Materials
The sample of pure chitosan was obtained as a gift from Yuhuan County Chemical Plant,
Zhejiang Province. Its degree of deacetylation is >82% and the molecular weight 2.0×105
2.2 Graft copolymerization and treatment of copolymer
Graft copolymerization was carried out in a 50mL four-necked flask equipped with thermometer, condenser, stirrer and gas inlet. In a typical reaction, 0.3 g chitosan was added with constant stirring under nitrogen. The required amount of monomer was added, followed by Ni (IV) aqueous solution and the total volume was made up to 20 ml with distilled water. The graft copolymerization was performed on the conditions of different temperature, monomer concentration, and initiator concentration. After completion of reaction, the reactant was cooled and neutralized by aqueous hydrochloric acid solution. It was poured into ethanol and the precipitated material was filtered through a weighed sintered glass funnel and washed over several times with ethanol. Then the crude graft copolymer was dried to a constant weight under vacuum at 60°C. The homopolymer was removed from the crude graft copolymer by extraction with THF for 48 hours. The final graft copolymer was dried at 60°C to a constant weight under vacuum.
2.3 Measurements
The graft copolymer chitosan- g- PAN was characterized by IR analysis using an FTS- 40 spectrophotometer in the potassium bromide medium. X- ray diffraction of the graft copolymer was carried out with Yaa 900 X- ray diffraction. The TGA of chitosan (4.3 mg) and the copolymers (4.22 mg) were carried out on a Shimadzu apparatus DGC-40 DTA-TG in atmospheric oxygen at a heating rate of 10°C/min.
3 RESULTS AND DISCUSSION
The grafting parameters, grafting percentage (G %), and efficiency percentage (E %), and the total conversion (C %) were defined and calculated as follows:
G % = (weight of PAN grafted/ weight of chitosan)×100 %
E % = (weight of PAN grafted/ total weight of PAN) ×100 %
C % = (total weight of PAN/ weight of AN charged) ×100 %
3.1 Effect of initiator concentration
When other reaction situations were kept unchanged, the effect of Ni (IV) concentration on
the extent of graft parameters is shown in Figure 1. The concentration of Ni (IV) effects
E % little, whereas the parameters G % and C % show the tendency of increasing first with
the increase of Ni (IV) concentration. This is because Ni (IV) attacks on the
characteristic group (- NH2) of chitosan backbone directly and increasing
macroradicals could be originated to initiate the graft copolymerization of chitosan and
AN. As a result, G % and C% augment. However, an excess of the Ni (IV) concentration may
accelerate the reaction of Ni (IV) and radical which terminates the chain propagating
reaction. At the same time, the chance of chain transfer reaction to monomer is enhanced.
All these can cause the decrease of grafting parameters consequently.
By studying the influence of the ratio of AN/chitosan on the graft yields, as shown in Figure 2, it is found that the grafting parameters reach a maximum when AN/chitosan is added up to 5.3 and then fall gradually. Because of the limited solubility of AN in the reaction medium, when the ratio of AN / chitosan is lower, the interaction between chitosan and AN is little so that both C % and G % is lower too. After the concentration of AN dissolved in water reaches saturation, the parameters reach maximum. When the ratio of AN/chitosan exceeds 5.3, the increase of AN volume results in the volume of water decline in the case the total volume keeps 20mL. Therefore, the amount of AN dissolved in water phase decreases and the concentration of Ni (IV) goes up, as explained above, which leads to the grafting parameters decrease finally.
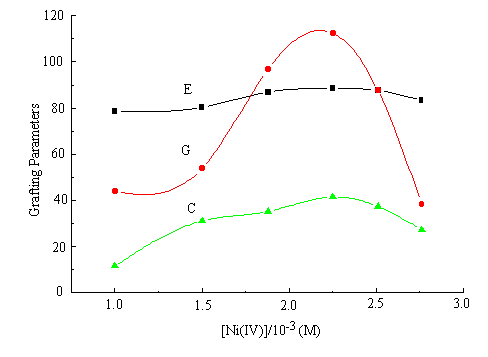
Fig.1 Effect of [Ni (IV)] on graft parameters
chitosan: 0.3 g; AN: 2.0 mL; 32°C; 2 h.
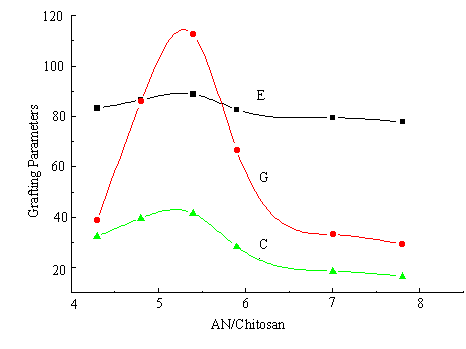
Fig.2 Effect of AN/Chitosan on graft parameters
chitosan: 0.3 g; [Ni (IV)]: 2.25×10-3 M; 32°C; 2 h. 3.3 Effect of the temperature
At the fixed concentration of AN, Ni (IV) and the amount of chitosan, the relationship between temperature and the grafting parameters has been investigated and is shown in Figure 3. Both C % and G % increase at first and then decrease rapidly. When the temperature of the reaction is lower, the grafting parameters increase with the increasing temperature. Whereas the grafting parameters level off when the temperature exceeds 32°C, which may be due to two factors mainly. On one hand, the chain transfer reaction speeds up. On the other hand, because the reaction is carried out in alkali medium, the hydrolysis of monomer is accelerated at higher temperature, which make the homopolymerization more easily [11]. As a result, the grafting parameters decrease sharply. The optimum temperature for maximum grafting is 32°C.
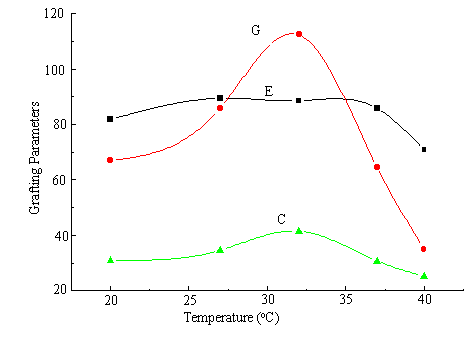
Fig.3 Effect of temperature on graft parameters
chitosan: 0.3 g; AN: 2.0 mL; [Ni (IV)]: 2.25×10-3 M; 2 h.
Fig.4 IR spectra. of chitosan (A) and chitosan-g-PAN (B).
3.4 IR SpectroscopyThe grafting was confirmed by comparing the IR spectra of chitosan with that of the grafted product. The results obtained are shown in Figure 4. The main difference observed is the presence of the intense absorption band at 2240cm- 1 in the IR spectra of the graft copolymer, which corresponds to the stretching frequency of nitrile groups (- C º N) of PAN chains. Moreover, the N- H zigzag vibration bands of the graft copolymer at 1660cm- 1 (I), 1560cm- 1 (II) compared with that of pure chitosan display the different characteristics obviously. So, it can be concluded that chitosan and AN are able to produce the graft copolymer with Ni (IV) as the initiator. In addition, owing to the results above, it could be proposed that Ni (IV) may react with amino group in chitosan to originate macroradicals first and then initiate AN grafting polymerization.
3.5 Thermal analysis
Thermogravimetric analysis (TGA) of pure chitosan and the grafted copolymer is shown in Figure 5. It is found obvious difference between them. The TGA of chitosan (A) shows a weight loss in two stages. The first stage ranges between 20°C and 107°C and shows about 13.08 % loss in weight. This may be corresponding to the loss of adsorbed and bound water. The second stage of weight loss starts at 197 °C and that continues up to 395 °C during which there was 40.55 % of weight loss due to the degradation of chitosan. Whereas the TGA of the grafted product (B) is different from it. It is observed the latter has three stages of distinct weight loss between 20 °C and 620 °C. The first stage ranges between 20 °C and 107 °C with 6.664 % of the adsorbed and bound water weight loss. The second stage of weight loss starts at188°C and that continues up to 350 °C during which there was 23.69 % of weight loss due to the degradation of ungrafted chitosan. There is 64.42% weight loss in the third stage from 350 °C to 620 °C that contributes to the decomposition of chitosan- g- PAN. So, it is evident that grafting acrylonitrile onto chitosan could enhance the stability of pure chitosan at higher temperature.
3.6 X- ray diffraction diagrams
The X- ray diffraction spectra of pure chitosan and chitosan- g- PAN were measured, which were shown in Figure 6. It is obvious that the X- ray diffraction spectra of the grafted chitosan increases one more peak than that of pure chitosan. One peak corresponds to the decreased crystallinity of pure chitosan, and the other corresponds to the crystallinity of grafted PAN on chitosan backbone. By calculation, it can be obtained that the crystallinity of chitosan is 0.3199 when the integrated angle range 2q is between 18.160° and 23.560° and that of chitosan- g- PAN is 0.2816 when 2q is between 15.520° and 23.560° . The crystallinity of the graft copolymer decreased obviously compared with chitosan due to the existence of PAN chain, which can destroy a part of crystal region of pure chitosan.
Fig.5 Thermogravimetric analysis (TGA) of pure chitosan (A) and the grafted copolymer (B).
Fig.6 The X- ray diffraction spectra of pure chitosan (A) and chitosan- g- PAN (B).
3.6 Solubility testThe solubility test of chitosan-g-PMA is carried out also. The grafted products are insoluble in solvents for either homopolymer. However, the graft copolymers swell in many solvents tested, such as 1% acetic acid solution, THF etc.
3.7 The initiation mechanism of grafting reaction
IR Spectroscopy, thermogravimetric analysis (TGA), X- ray diffraction diagrams and solubility test all illustrate that AN has been grafted onto chitosan. It is verified once more that the process Ni (IV) -> Ni (II) belongs to the two- step single electron transfer mechanism [11]. The IR spectrum above has revealed that the N- H zigzag vibration bands of the graft copolymer at 1660cm-1(I), 1560cm-1(II) compared with that of pure chitosan display the different characteristics obviously. So, it could be proposed that Ni (IV) may react with amino group in chitosan to originate macroradicals first and then initiate AN grafting polymerization. The initiation mechanism, referencing to the related papers [11,20], may be shown as follows:
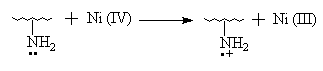
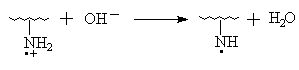
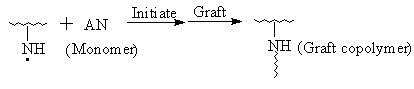
and again
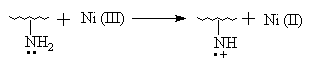
etc.
4 CONCLUSION
The feasibility of grafting acrylonitrile onto chitosan by using Ni (IV) - chitosan as the redox initiator has been demonstrated by this work. The grafted products can enhance the stability of pure chitosan at higher temperature and are insoluble in solvents for either homopolymer. However, the graft copolymers swell in many solvents tested, such as 1% acetic acid solution, THF etc. The crystallinity of the graft copolymer decreased obviously compared with chitosan due to the existence of PAN. The compatibility of chitosan and PAN, that is poor naturally, is increased by means of the graft copolymerization and the graft copolymer is expected to be high absorbent resin after being hydrolyzed.
Under the experimental conditions, graft copolymer with high graft efficiency and grafting percentage using Ni (IV)- chitosan system as initiator has been obtained. Ni (IV)- chitosan is concluded to be an efficient redox initiator for the graft copolymerization. The two- step single electron transfer mechanism proposed in redox process is a modification to traditional mechanism. Moreover, because the activation energy of the reaction employing Ni (IV)- chitosan as initiator is low so that the graft copolymerization is able to be carried out at a mild temperature 32 °C, which is superior to other initiators. So, Ni (IV)- chitosan as initiator is thought to be practical and has a good prospect.
REFERENCES
[1] Gao Jianping, Tian Ruchuan, Chang Liming. Chin. J. Polym. Sci., 1996, 14 (2): 163-171.
[2] Gao Jianping, Yu Jiugao, Wang Wei et al. J. Macromol. Sci., Pure Appl. Chem.,
1998, A 35 (3): 483-494.
[3] Liu Yinghai, Liu Weihong, Yang Jiming et al. J. Molecular Sci., 1996, 12 (2): 190-194.
[4] Liu Yinghai, Song Xingru, Shi Hongmei. Chem. J. Chinese Univ., 1990, 11 (3): 328-330.
[5] Murthy C P, Sethuram B, Rao T. Navaneeth. Z. Phys. Chem. (Leipzig) 1986, 267 (6):
1212- 1218.
[6] M Afzal Ali Siddiqui, C Sudheer Kumar, U Chanddraiah Sushama Kandlikar. Indian J.
Chem., 1991, 30 (A): 849-854.
[7] G H Hugar, S T Nandibewoor. Indian J. Chem. 1993, 32A: 1056-1059.
[8] Wang A Z, Li F M, Ding T H. Chem. Res. Chinese Univ., 1992, 8 (4): 432?438.
[9] Li Z T, Wang F L, Wang A Z. Int. J. Chem. Kinetics, 1992, 24: 933-941.
[10] Shang Y J, Liu Y H, Deng K L. J. Hebei Univ., 1999, 19 (4): 356.
[11] Liu Y H, Shang Y J, Li W P et al. Acta Polymerica Sinica, 2000, (2): 235.
[12] Shantha K L, Bala Udaya, Rao K Panduranga. Eur. Polym. J., 1995, 31 (4):
377-382.
[13] Kim Kyoung Ho, Kim Kong Soo, Kim Yong Beom. Pollimo, 1987, 11 (30): 261-267.
[14] Ouchi Tatsuro, Banba Toshio. J.Macromol. Sci., Chem., 1991, A 28 (10): 959.
[15] Kim Chun Ho, Jo Sung-Kwan. Kong op Kwahak, 1995, 6 (2): 267.
[16] Kang?Doo Whan, Choi Hoo Rak, Kweon Dong Keon. J. Appl. Polym. Sci., 1999, 73 (4):
469-476.
[17] Chern Chorng-Shyan, Lee Cheng-Kang. J. Polym. Sci., Part A: Polym. Chem., 1999, 37
(10): 1489.
[18] Tang X R, Cao Y H, Wang XY, et al. Natural Product R. & D., 1997, 9 (1): 86-91.
[19] Crouthamel C E, Meek H V, Martin D S. J. Am. Chem. Soc., 1949, 71: 3031.
[20] Liu Y H, Li W P, Deng K L et al. J. Appl. Polym. Sci., 2001, 82 (11): 2636-2640.