http://www.chemistrymag.org/cji/2002/047033pe.htm |
May 1,
2002 Vol.4 No.7 P.33 Copyright |
Shen Shigang, Cui Yuping, Sun Hanwen, Shan
Jinhuan, Liu Ying
(College of Chemistry & Environmental Science, Hebei University, Baoding 071002,
China)
Received Feb.
18, 2002.Abstract This work was primarily an
experimental investigation of the bromate oscillator using glycerol and acetone as the
mixed organic substrates. The empirical equation of the induction period and oscillating
period with the concentrations of the reactants and temperature were obtained. The
oscillating characteristic and possible oscillation mechanism were analyzed.
Keywords Oscillation, Glycerol, Acetone, Mixed substrates.
Chemical oscillating reactions represent a typical far-from-equilibrium phenomenon in a system that the concentrations are not uniform in the course of time. It is also called as the chemical clock, which is similar to the biological clock. The concentration of some substances in the reaction system exhibits a regular change with time, a kind of self-regulating function similar to what happened in an organism. The study of life assimilation and activation intermediate involved in the oscillating system during the metabolic process provides important information and theoretical basis in the sugar fermentation in organism in imitating organism. During the last twenty years, Vitamin C, glucose, pyruvic acid, fructose, amino acid, lactose, and malic acid have been reported[1-8] to be very important reaction substrates in oscillating reaction, they play an important part in the synthesis of cell substances in life[9]. As a dehydrator and lubricator, glycerol has been used clinically in curing many diseases. In the present work, a new experimental investigation of the bromate oscillator using glycerol and acetone as the mixed organic substrates was reported. The temperature effects on the oscillations of this system and the initial concentration range of the reactants in the oscillating system were examined. The relations of the oscillation periods with the substrate concentrations have been studied in detail. The oscillating characteristic and a possible oscillation mechanism were analyzed.
2 EXPERIMENTAL
2.1 Reagent
Analytical grade glycerol, manganous sulfate, sulfuric acid, acetone were used in the
experiments. Analytical grade potassium bromate was recrystallized in water. The water
used in the experiments was double deionized.
2.2 Apparatus and method
Experiments were performed in a self-made thermostat (The temperature was controlled at T¡À 0.1 K). The overall volume of the solution is 50 ml. Sulfuric acid, glycerol, manganous sulfate and acetone were added successively under stirring by a magnetic agitator. When the temperature of the reaction mixture reached constant (¡À 0.1 K), the solution of potassium bromate thermostated at the same temperature was instantly transferred into the mixture. The oscillating curves of the potential with time were recorded using a table x-t recorder. Smooth bright platinum was employed as indicator electrode and a 217-Type SCE was used as the reference electrode together with (1 mol dm-3 sulfuric acid) liquid junction having a sintered silica disc at the end dipping in the reaction mixture. Since only the characteristic of the oscillating curve with time was considered in the present work, the typical trace of potential oscillations was shown in Figure 1.
3 RESULTS AND DISCUSSION
3.1 Oscillating phenomenon
Table 1 gives the initial concentration range of the reactants and temperature range in the chemical oscillating system of Glycerol-Acetone-Bromate-MnSO4-H2SO4.
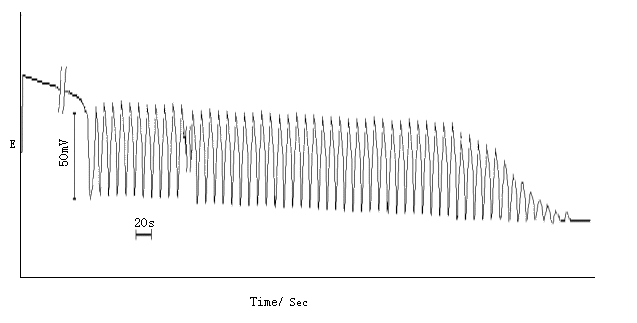
Fig.1 The oscillating curve of the potential with time
[System: [Act]0 = 1.35 mol dm-3, [GL]0 = 3.30¡Á 10 -2 mol dm-3, [BrO3-]0 = 0.090 mol dm-3, [Mn2+]0 = 6.0¡Á10-4 mol dm-3, [H2SO4]0 = 2.40 mol dm-3, Temp. = 298¡À 0.1 K]
Table 1. The initial concentration range of the reactants and temperature range in the oscillating system
| [Act]0 · mol-1dm3 |
[GL]0· mol-1dm3 |
[BrO3- ]0· mol-1dm3 |
[Mn2+]0´104· mol-1dm3 |
[H2SO4]0· mol-1dm3 |
T/K |
| 0.27-2.43 | 0.0330 |
0.090 | 6.0 | 2.40 |
298 |
| 1.35 | 0.0110-0.0550 |
0.090 | 6.0 | 2.40 |
298 |
| 1.35 | 0.0330 | 0.066-0.114 | 6.0 |
2.40 |
298 |
| 1.35 | 0.0330 | 0.090 | 1.20-14.40 |
2.40 |
298 |
| 1.35 | 0.0330 | 0.090 | 6.0 | 1.90-2.88 |
298 |
| 1.35 | 0.0330 | 0.090 | 6.0 | 2.40 |
293-323 |
In the concentration range
listed in Table 1, the color of the solution changed from pink (the color of Mn2+)
to brown, and at the same time the potential of the system went up rapidly after adding
KBrO3 solution as required by the experiment. After a period of induction, the
potential decreased rapidly, and the system exhibited a periodic oscillation between brown
and pale yellow. In the latter stage, the solution color became light, and the oscillating
period did not change much during the whole oscillating process. With the duration of time, the oscillating amplitude began to show a trend of
slow increase, and after a period of stable amplitude, the amplitude began to decrease
gradually until the oscillation stopped.
3.2 The effect of temperature and the apparent activation energies
The Glycerol-Acetone-Bromate-MnSO4-H2SO4 chemical
oscillating system is very sensitive to temperature changes. When the temperature
increased, the induction period ti(s) and oscillating period tp(s)
(the third oscillating period was used in the paper) decreased regularly.
The composition of the reaction mixture was as follows: [Act]0
= 1.35 mol dm-3, [GL]0 = 3.30
ln(ti-1s) = -Ei/RT + Ai
ln(tp-1s) = -Ep/RT + Ap
in which Ai and Ap are the intercept of the lines, Ei/R and Ep/R are the slopes of the fitting lines, respectively. Compared with the Arrhenius Equation ln(k) = -EA/RT + A, ti-1 and tp-1 are very similar to the reaction rate constants, but Ei and Ep should be corresponding to the activation energies, which are called the apparent activation energies in this paper, and their values are Ei = 69.7 kJ mol-1 and Ep = 72.0 kJ mol-1 respectively. The results are in good agreement with the data in the literature[10].
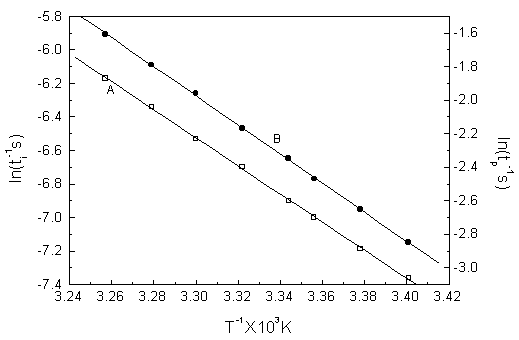
Fig.2 The figure of ln(t-1s) with T-1K
A: ln(ti-1s) with T-1K; B: ln(tp-1s) with T-1K.
[System: [Act]0 £½ 1.35 mol dm-3, [GL]0 £½ 3.30¡Á10 -2 mol dm-3, [BrO3-]0£½ 0.090 mol dm-3, [Mn2+]0 £½ 6.0¡Á10-4 mol dm-3, [H2SO4]0 = 2.40 mol dm-3.]
3.3 The effects of the reactant concentration
It is found from the experiment that both the induction period ti(s) and the
oscillation period tp(s) of the chemical oscillating reaction were affected by
the concentrations of the reactants, ln(t
ln(t i/s) = ai + bi ln([Act]0 mol-1 dm3) + ci ln([GL]0 mol-1 dm3) + di ln[BrO3- ]0 mol-1 dm3) +ei ln([Mn2+]0 mol-1 dm3) + fi ln[H2SO4]0 mol-1 dm3)
ln(tp/s) = ap + bp ln([Act]0 mol-1 dm3) + cp ln([GL]0 mol-1 dm3) + dp ln[BrO3- ]0 mol-1 dm3) + ep ln([Mn2+]0 mol-1 dm3) + fp ln[H2SO4]0 mol-1 dm3)
Based on the experimental data (which are composed of 45
experimental data, each represents the average of three parallel experimental results),
the correlation coefficients in the above two formulae can be determined by plural linear
fitting: ai = 5.80, bi = -0.48, ci =-1.01, di
= 0.71, ei =- 0.21, fi = -2.24; ap = 6.64, bp
= -0.63, cp = -0.99, dp = 0.99, ep =0.57, fp
=-0.86. The plural linear correlation coefficients are 0.999 and 0.998, respectively.
Therefore, the relationship of the induction period t i(s) and oscillating
period tp(s) with the initial concentration of the reactants can be expressed
as:
Ti(s) = 332[Act]0-0.48[GL]0-1.01[BrO3-
]00.71[Mn2+]0-0.21[H2SO4]0-2.24(mol
dm-3)3.23s
Tp(s) = 768[Act]0-0.63[GL]0-0.99[BrO3- ]00.99[Mn2+]00.57[H2SO4]0-0.86(mol dm-3)0.92s
It can be seen from the above relationship, that increasing [Act]0, [GL]0, and [H2SO4]0 can increase the rate and shorten induction period ti(s) and oscillation period tp(s); Increasing [Mn2+]0 can shorten the induction period ti(s) and elongate the oscillating period tp(s); Increasing [BrO3-]0 can elongate the induction period ti(s) and oscillating period tp(s).
3.4 The function of acetone
While keeping other reaction conditions unchanged, water was added in place of acetone .
It was found that adding potassium bromate solution produced large quantity of Br2.
The system color changed to brown and gave off a brown gas. After the induction period,
the potential decreased without going up, and the brown color did not disappear, and no
oscillating reaction took place. The existence of an induction period demonstrates that
the accumulation process of HBrO2 exists in the oscillating system. In acidic
medium, under the catalytic condition of Mn2+, an oxidation-reduction reaction
takes place between BrO3- and GL producing Br2 which
caused the solution color to change to brown. The existence of large amount of Br2
prevented the accumulation of HBrO2. The brown color disappeared quickly after
adding acetone and the oscillation took place. When nitrogen was rapidly bubbled through
the system with no acetone added, some oscillations could be observed. So one of the main
function of acetone is to eliminate excessive Br2 and to produce Br-
simultaneously, i.e.,
Br2+CH3COCH3![]() CH3COCH2Br+Br-
+H+ (1)
CH3COCH2Br+Br-
+H+ (1)
Br- can be oxidized by BrO3-, which is
favorable for the accumulation of HBrO2, so this is an oscillation switch.
Increasing the amount of acetone increased the accumulation of HBrO2, and the
induction period became shorter. This accelerated the whole oscillating process, and
Keeping the other conditions constant, adding small amount of bromoacetone shortened the induction period. It indicates that the accumulation of bromoacetone is very important during the induction period. Bromoacetone was partly oxidized by Mn3+ to produce Br-, which was favorable for the accumulation of HBrO2 and the induction period was shortened. The reaction process is as follows:
Mn3++CH3COCH2Br
3.5 The function of GL
GL played the role of the reductant and participated in the formation of Br2 at the same time. So with the increase of the initial concentration of GL, the rate of regeneration of Mn2+ through the reduction of Mn3+ by GL increased, and the induction period ti(s) and oscillating period tp(s) became shorter.
3.6 The function of Mn2+
If replacing Mn2+ by water, though no oscillation occurred, the solution color changed to light-brown gradually. This indicates that though there is no Mn2+ as catalyst, BrO3- can partly oxidize GL. This is because alcohol is more easily oxidized than acid[6,7]. This reaction is shown as processes E which is the side reaction of the oscillating reaction, it consumes partial GL. In the presence of Mn2+, BrO3- and GL reacted rapidly to produce Br2, so that the color of the solution changed rapidly to brown. Here Mn2+ plays the catalytic role, and the catalytic process is shown as follows:
BrO3-+Mn2++5H+
Mn3++GL
HOBr+GL
________________________________________________________________
BrO3-+2GL+5H+
Br2 produced was removed in Reaction (l) to form Br- at the same time. The Br- thus formed can be oxidized by BrO3-which is favorable for the accumulation of HBrO2.The reaction process11 took place as Processes A as follows.
At the beginning of the reaction (the induction period), the concentration of Br- was very low. The catalytic process took place predominantly. With the increase of the concentration of Mn2+, it might cause Reaction (3) and (5) to proceed very fast and more Br2 was produced. This further caused Reaction (1) to produce more Br- and then Reaction (6) accelerated the accumulation of HBrO2. The time to reach the critical value of the oscillating was very short, so the induction period ti(s) became shorter with the increase of [Mn2+]0. At the beginning of the oscillation, Mn3+ returned to Mn2+ through Reaction (4) and (2). When [Mn2+]0 was in excess, though the rate of Reaction (4) became faster, every oscillation reaction consumed large amount of GL, causing [GL] to become lower; and during each period the time of Mn3+ returning to [Mn2+] needed became longer with the increase of [Mn2+]0; So the oscillating period tP(s) became longer with the increase of [Mn2+].
3.7 The function of BrO3-
If without the catalysis of Mn2+, BrO3- can still oxidize part of GL and consumes part of GL, which shown as Process E. So with increasing the concentration of BrO3-, more GL was consumed. Therefore with the increase of [BrO3- ], the induction period ti(s) and the oscillating period tP(s) became longer.
3.8 The effect of the radical inhibitor
Keeping other conditions constant, when acrylonitrile was added into the reaction system which was oscillating, the oscillation stopped immediately, or when ethanol was added into the reaction system which was oscillating, the oscillation stopped immediately after a few oscillation. Since acrylonitrile and ethanol are both radical inhibitors, it indicates that radicals have been involved in the oscillation reaction. It is reported [11] that the possible radical reaction process may take place as Processes B in the following.
3.9 Discussion on the oscillating mechanism
Based on the above discussions, it is proposed that the system may have undergone the following five processes[11,12,13]:
Process A: Br-+BrO3-+2H+
HBrO2 + Br-+ H+
5 Br-+ HOBr + H+
________________________________________________________________
5Br-+BrO3-+H+
Process B: 2HBrO2![]() HOBr+BrO3-+2H+ (9)
HOBr+BrO3-+2H+ (9)
2(HBrO2+BrO3-+H+![]() 2BrO2·+H2O)
(10)
2BrO2·+H2O)
(10)
4(BrO2·+Mn2++H+![]() Mn3++HBrO2)
(11)
Mn3++HBrO2)
(11)
_________________________________________
BrO3-+4Mn2++H+![]() 4Mn3++HOBr
(III)
4Mn3++HOBr
(III)
Process C: Mn3+ returned
to Mn2+ through Reaction (4) and (2)
Process D: Br- was regenerated by Reaction
(1) and (2)
Process E: BrO3- oxidized GL through Reaction
(6), (7) and (5). The result reaction is shown as follows:
2Br-+BrO3- +GL![]() 3/2Br2+ 3Oxidation Product III (IV)
3/2Br2+ 3Oxidation Product III (IV)
The existence of the induction period indicates that the mechanism is
an automatic catalytic process dominated by BrO2· produced in
Reaction (10) during Process B. The catalyst is HBrO2. Br- plays the role of kinetic control. The multitude of [Br- ] determined the rate of the self-catalytic oxidization. When [Br-] concentration is large enough, Process A dominates, and Process B
is inhibited, which caused the formation rate of BrO2 to become
slower. The outcome of Process A is to make Process B dominating by removing Br- from the system. The proceeding of Process B results in
[Mn3+] in Reaction (11) increasing constantly, which causes Process C to
proceed leading to the regeneration of Mn2+. At the same time, Br- is formed through Process D. These processes go round and
round, the oscillating phenomenon of [Br-] or [Mn3+]/[Mn2+]
begins to take place and repeatedly performed.
[1] Zhao X Z, Zhao H X, Xu Y T et al. Chemistry (Huaxue Tongbao), 1985, 30: 586.
[2] Li H X, Liu M, Wang S X et al. Acta Physico-Chimica Sinica, 1990, (6): 609.
[3] Lu C H, Xu Z Q, Xu J D et al. Chemistry (Huaxue Tongbao), 1992, (2): 30.
[4] Shen S G, Zhao J G, San J H et al. J. Hebei University, 1995, 5: 21.
[5] Yuan C L, Li Z X, Wang J C. Acta Physico-Chimica Sinica, 1994, 10: 87.
[6] An C J, Zhuang L, Liu Y et al. Acta Chimica Sinica, 1997, 55: 259.
[7] An C J, Gan N Q, Liu Y et al. Acta Chimica Sinica, 1998, 56: 973.
[8] Rastogi R P, Verma M K, Yadav R D. Indian J. Chem., 1985, 24A: 721.
[9] Trivett T L, Meyer E A. J.Bacteriol., 1971, 107: 770.
[10] Pastapur S M, Kulkarni V R. J.Indian Chem. Soc., 1991, 88: 293.
[11] Field R J, Burger M. Oscillations and travelling waves in chemical system. Wiley-interscience, New York, 1984.
[12] Field R J, Koros E, Noyes R. J. Am. Chem. Soc., 1972, 94: 8649.
[13] Noyes R M. J. Am. Chem. Soc., 1980, 102: 4644. ¡¡
¡¡