http://www.chemistrymag.org/cji/2002/047034ne.htm |
May 1,
2002 Vol.4 No.7 P.34 Copyright |
Determination of Cu, Zn, Fe, Ni and Pb in europia by ICP-AES after preconcentration by saccharomycete immobilized on silica gel
Fan Zhefeng, Jin Xiaotao(Center of Analysis and Test; Shanxi Normal University, Linfen 041004)
Abstract A method for the
determination of Cu, Zn, Fe, Ni and Pb by inductively coupled plasma emission spectrometer
after preconcentration on a column containing saccharomycete immobilized on silica gel has
been developed. Optimum pH valves, amount of adsorbent, elution solution and flow rate has
been obtained for the elements studied. Recoveries of the elements studied were between
98.3 and 101%. The adsorption capacity was 5.7, 9.8, 6.9, 9.3 and 8.2 mg.g-1
for Cu, Zn, Fe, Ni and Pb, respectively. The proposed method has been successfully applied
to the determination of trace metals in europia (GBW 02902). Trace metals have been
determined with relative error lower than 5%.
Keywords Trace metals determination, preconcentration, saccharomycete, silica
gel, ICP-AES.
1 INTRODUCTION
Biological organisms can accumulate or
preconcentrate heavy metals from aqueous solutions, this was called biosorption that occur
a process in which metal ions are adsorbed on the surface of the organisms through
interactions with chemical functional [1,2]. In recent years, immobilized
organisms are being widely used because of the advantages for on-line matrix isolation in
flow analysis, low resistance to fluid flow, self-supporting rigidity, excellent
durability easy regeneration and recovery of metals [3]. In many studies,
controlled-pore glass [4,5] and silica gel [6,7] have been used as a
substrate for the immobilization of algae cells and bacteria such as Escherichia coli and
Pseudomonas putida.
In this paper, conditions for the preconcentration of Cu, Zn, Fe,Ni and Pb in europia by saccharomycete
immobilized on silica gel and subsequent determination by inductively coupled plasma
emission spectrometer (ICP-AES) have been studied in detail for the first time.
Experiments showed that the method presented in this work was not only high sensitive but
also easy to operate and has good selectivity. This method has been applied to determine
trace metals in europia.
2.EXPERIMENTAL
2.1 Apparatus and reagents
Atomic scan 25 inductively coupled plasma
emission spectrometer (Thermo Jarrell Ash Co.), RF power 1150 W, observation height 15 mm,
coolant gas flow rate 20 L. min-1, plasma gas flow rate 0.5 L.min-1,
carrier gas flow rate 0.5 L.min-1. Analytical lines were of Cu 324.754nm, Zn
213.856 nm, Fe 238.204 nm, Ni 221.647 nm and Pb 220.353 nm, respectively. All pH
measurements were performed with a pHS-3TC model digital meter (Shanghai Tianda Co.).
All chemicals used for preparation of solutions were of analytical
grade reagent. All metals stock solutions (1000 mg.mL-1) were prepared by dissolving the
appropriate amounts of metals with doubly distilled water. The working solutions were
prepared by appropriate dilution of the stock solutions immediately prior to their use.
Saccharomycete from the National institute of biochemistry, silica gel (60 mesh),
double-distilled water (18.3 MW.cm-1) was used for the preparation of dilute
solutions.
2.2 Preparation of Saccharomycete on silica gel
Dry saccharomycete (0.2 g) was mixed with 2 g of silica gel. The mixture
was wetted with 2 mL of double-distilled water and thoroughly mixed. After mixing, the
paste was heated in an oven at 80℃for 24 h to dry the mixture. The wetting and drying
step was repeated to maximize the contact between saccharomycete and silica gel, thereby
improving the immobilization efficiency. Then, the silica gel-bacteria briquette was
broken to get original size (60 mesh).
2.3 Column preparation and procedure for
preconcentration
Saccharomycete immobilized on silica gel (0.25g) was packed in a glass
column (0.5 cm i.d. and 20 cm length). Before use, 1 mol L-1 HNO3
solution and double-distilled water were passed through the column, in order to condition
and clean it. Then, the column was conditioned to pH 5.5, 100 mL of metal ion solution
containing 0.5 mg of Zn, 2.5 mg of Cu, Ni and Pb, 2.0 mg of Fe was taken and the pH was
adjusted to 5.5 with nitric acid or ammonia. The resulting solution was passed through the
column. By using a peristaltic pump, the flow rate was adjusted to 2.0 mL.min-1.
In order to elute the retained metal ions from saccharomycete on silica gel, 10 mL 1 mol L-1
of HCl and HNO3 solutions in the ration of 1+3(v/v) were used. The
concentration of trace metal ions was determined by ICP-AES. The saccharomycete on silica
gel was used repeatedly after washing with 1 mol L-1 of HNO3 solution
and distilled water, respectively.
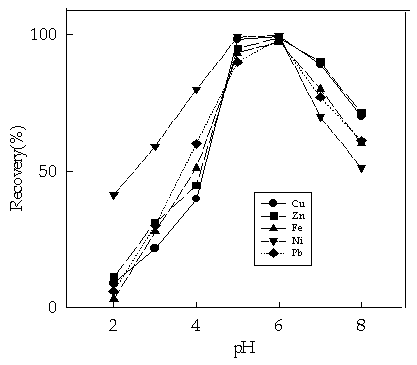
Fig. 1 The effect of pH on recovery of the
metal ions studied
(sample volume, 100mL; flow rate 2.0 mol min-1; Zn 0.5mg; Cu, Ni and Pb, 2.5mg; Fe, 2.0 mg)
3 RESULTS AND DISCUSSION
3.1 Effect of pH
The pH of the sample solution directly
affects recovery of the metal ions, For this purpose, the pH values of element solutions
were adjusted to a range of 2 to 8 with nitric acid or ammonia. As shown in Fig.1. The
optimum pH of the sample solution for the retention is 5.5 for all metal ions studied.
3.2 Effect of amount of adsorbent
The retention of the elements is depended
on the amount of adsorbent used. For that reason, the effect of the amounts of adsorbent,
which was varied from 0.1 to 0.4 g was investigated. It was found that above 0.2g of
adsorbent the recovery of Zn and Ni was gradually increased, while at about 0.25g of
adsorbent that of all elements reached a plateau. Therefore, 0.25 g of adsorbent was found
to be optimum of all preconcentration purposes.
3.3 Effect of type, volume of elution solutions
The other important factor that affects the
preconcentration technique is the type, volume and concentration of the elution used for
the release of metal ions from the column. The concentration of the acid used as an
elution must be the lowest possible level in order to prevent the biomass from
degradation. Hydrochloric acid (0.5 mol L-1) and nitric acid (1 mol L-1)
solutions and the mixture of 1 mol L-1 hydrochloric acid and nitric acid
solutions in the ration of 1+3(v/v) were tested. The elute volume was 5,10 and 15mL. The
results showed that 10 mL of the mixture acid were found to be satisfactory for the
quantitative elution metal ions studied (recovery >95%).
3.4 Effect of flow rates of sample solutions
The flow rate of the sample solution affects
the mass transfer from the solution to the binding sites on the cell wall of
sccharomycete. For that reason, The flow rate of the sample solution was examined under
optimum conditions (pH, elute type) by using peristaltic pump. The solution was passed
through the column with the flow rates adjusted in a range of 0.5-5.0 mL.min-1.
As shown in Fig.2. The optimum flow rate was 2.0 mL.min-1. Therefore 2.0 mL min-1
was used as a flow rate for the experiments.
3.5 Capacity
The adsorption capacity of saccharomycete on silica gel is investigated under the
operating conditions. The adsorption capacities were found as 5.7, 9.8, 6.9, 9.3 and 8.2 mg.g-1 for Cu、Zn、Fe、Ni and
Pb, respectively.

Fig. 2 The effect of flow rate of sample solution on recovery of the
metal ions studied
(sample volume, 100mL; flow rate 2.0 mol min-1; Zn 0.5 mg; Cu, Ni and Pb, 2.5 mg; Fe, 2.0 mg)
3.6 Detection limit and precision of the
method
The detection limit (evaluated as the concentration
corresponding to three times the standard deviation of the blank signal) of the method
were found to be 2.7 ng.mL-1 for Zn, 2.5 ng.mL-1 for Cu, 1.4 ng.mL-1
for Fe, 2.9 ng.mL-1 for Ni and 56 ng.mL-1 for Pb. The relative
standard deviation is 1.8 to 4.2% for 0.20
4 APPLICATION
The proposed preconcentration method were
applied to the determination of Cu, Zn, Fe, Ni and Pb in standard europia reference
material (GBW02902). Standard europia sample (0.2 g) was weighed accurately, dissolved and
diluted in 100 mL of volumetric flask, pH was adjusted to 5.5 with HNO3 or
ammonia. Cu, Zn, Fe, Ni and Pb were determined after applying the general preconcentration
procedure described above. The results are given in Table 1. It is clear that the
obtained values for both metals agreed very well with the certified values.
Table 1 Determination of standard europia reference material (GBW 02902)(n=5)
Element |
Certified |
Found |
RSD |
Recovery |
Cu |
5.35 |
5.30 |
1.8 |
99.1 |
Zn |
12.53 |
12.45 |
2.0 |
99.7 |
Fe |
5.04 |
5.07 |
2.4 |
101 |
Ni |
7.54 |
7.41 |
3.1 |
98.3 |
Pb |
6.93 |
6.89 |
4.2 |
99.4 |
[1] Madrid, Camara C, Trends. Anal.Chem, 1997, 16: 36.
[2] Aller A J, Robles L C. J. Anal. At spectrom, 1998, 13: 469.
[3] Maquieira A, Elmahadi H A M, Puchades R. J. Anal. At spectrom, 1996,11: 99.
[4] Maquieira A, Elmahadi H A M, Puchades R. Anal, Chem, 1994, 66: 1462.
[5] Maquieira A, Elmahadi H A M, Puchades R. Anal, Chem, 1994, 66: 3632.
[6] Mahan C A, Holcombe J A. Anal, Chem, 1992, 64: 1993.
[7] Robles L C, Aller A J. Anal, 1996, 15: 21.