http://www.chemistrymag.org/cji/2002/048039ne.htm |
May 31,
2002 Vol.4 No.8 P.39 Copyright |
Zhu Jing, Gao Yang, Cao Shaokui*, Zhang
Wennan#, Wang Dongmei#, Cui Huifang#, Li Tianxuan#
(College of Materials Engineering, Zhengzhou University, Zhengzhou 450052; #Henan
Provincial Key Laboratory of Fine Chemicals, Zhengzhou 450002)
Received Apr. 8, 2002.
Abstract Some symmetric and asymmetric
meso-hydroxyl substituted tetraphenylporphyrins were synthesized by using a modified Adler
method. The modification was to add an anhydride compound into the reaction system in
order to timely remove water produced in the reaction process. Through the modification,
the yields of four hydroxyl groups substituted meso-tetraphenylporphyrins (THPPs), the
symmetric products, were in the range of 14-22%, and the yield of the asymmetric product
containing only one hydroxyl group was higher than 10%.
Keywords tetraphenylporphyrin, modified Adler method, hydroxyl substitution
Meso-hydroxyl substituted
tetraphenylporphyrins and their derivatives are not only useful and highly sensitive
chromogenic agents in analytical chemistry [1,2], but also important
intermediates in the preparation of bio-mimetic catalyst in imitating photosynthetic
charge separation [3]. And also symmetric THPPs are useful precursors for
porphyrin-containing liquid crystal assemblies[4-8] and man-made oxygen carrier
with the similar function of heme [9,10].
Adler method was advanced by Alan D. Adler et al. in 1967, which is
producing porphyrin in one step by adding equal molar pyrrol and aldehyde into propionic
acid or acetic acid [11]. The method was suitable for many
meso-substituted porphyrins and had become classical method. However, if the substituted
groups at the meso-phenyl position were strong polar groups, such as OH, NO3,
or NH3. The yields of porphyrins would be very low, even be zero,
and the product would contain a lot of black insoluble impurities. Bearing strong polar
hydroxyl groups, THPPs were normally prepared in a very low yield by Adler method and
other methods. For example, the symmetric THPPs were synthesized through alkoxy hydrolysis
[12,13] with a yield of 4.8% for 4-position hydroxyl substitution [T(4-HP)P],
and 9.3% for 2-position hydroxyl substitution [T(2-HP)P]; diazo hydrolysis gave only a
yield of 4.3% for T(4-HP)P and 3.7% for T(2-HP)P [14,15]; the yield of T(4-HP)P
was about 10% through the alkali fusion of hydrosulfonic acid [15] and the
direct ring formation [16]; the yields of asymmetric single hydroxyl
substituted meso-tetraphenylporphyrin by the above four methods were even lower than 10%.
We found a modification of the Adler method can greatly improve the
yield of THPP by adding an anhydride compound into the reaction system in order to timely
remove water produced in the reaction process (as shown in Scheme 1) . With such a
modification, the yields of the symmetric product THPPs were in the range of 14-22%; and
that of the asymmetric product containing only one hydroxyl group was higher than 10%.
These yields were much higher than the reported results so far. The modified Adler method
provides an easy and convenient alternative to the existent method in getting higher THPP
yield with high purity.
1 EXPERIMENTAL
Meso-tetra(4-hydroxyphenyl) porphyrin [T(4-HP),4a]
To a solution containing 18.4 g (150 mmol) of 4-hydroxy-benzaldehyde in 300ml of propionic
acid, 14 ml (106 mmol) of propionic anhydride were added. The resulting solution was
allowed to reflux with mechanical stirring under the protection of bubbled nitrogen. Then,
10.5 ml (150 mmol) of freshly distilled pyrrole in 10 ml of propionic acid was added
dropwise. After the addition, the reaction mixture was stirred for further 30 min, then
300 ml of 95% EtOH was added under vigorous stirring. The mixture was cooled to room
temperature, and then stored at ¨C15oC
overnight. The tarry mixture was filtered and the solid product was washed repeatedly with
a mixture of EtOH and propanoic acid (1:1 in volume), then with hot water until the rinse
solution were no longer dark. The filter cake was dried in air overnight, then at 150oC
for 2h. The purple crystalline 4a was received with a
yield in a range of 19-22%. 1H-NMR (300MHZ, Acetone-d6,d in ppm): 8.92 (8H, pyrrole), 2.80 (4H,
hydroxyl), 7.28~7.31 and 8.04~8.08 (16H, phenyl). IR (KBr tablet, cm-1):
3435.0, 1607.1, 1509.3, 1349.7, 1264.5, 1170.4, 1103.1 and 802.4. UV/VIS (lmax, nm in EtOH): 419.5 (the soret
band), 516.0, 556.0, 597.0 and 649.0 (the Q band). Elemental Analysis, found (calculated
for C44H30N4O4 ):C 77.69(77.86), H 4.62(4.44),
N 8.26(8.33).
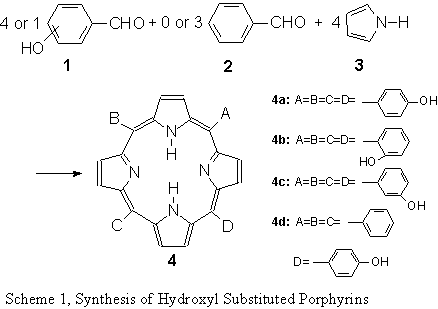
Meso-tetra(2-hydroxyphenyl) porphyrin [T(2-HP),4b]
Meso-tetra(2-hydroxyphenyl)-porphyrin 4b was prepared in a similar manner as described for the para isomer 4a with a yield of 20%. 1H-NMR (300MHZ, Acetone-d6, d in ppm): 8.95 (8H, pyrrole), 3.01 (4H, hydroxyl), 6.92~7.10 and 8.01~8.04 (16H, phenyl). IR (KBr tablet, cm-1): 3529.1, 1591.7, 1509.3, 1349.7, 1225.5, 1170.3 and 798.7. Elemental Analysis, found (calculated for C44H30N4O4) : C 77.44(77.86), H 4.76(4.44), N 8.30(8.33).
Meso-tetra(3-hydroxyphenyl)-porphyrin [T(3-HP),4c]
Meso-tetra(3-hydroxyphenyl)-porphyrin 4c was prepared in the similar way as 4a with yield of 14%. 1H-NMR (300MHZ, Acetone-d6, d in ppm): 8.90 (8H, pyrrole), 2.91 (4H, hydroxyl), 7.20~7.51 and 8.01~8.06 (16H, phenyl). IR (KBr tablet, cm-1): 3405.0, 1608.7, 1599.7, 1347.4, 1232.2. Elemental Analysis, found (calculated for C44H30N4O4): C 77.40 (77.86), H 4.80 (4.44), N 8.23(8.33).
5-(4-hydroxyphenyl)-10,15,20-triphenyl porphyrin [5-(4-HP)P, 4d]
5-(4-hydroxyphenyl)-10,15,20-triphenyl porphyrin 4d was prepared in the similar way as 4a with 120 mmol freshly distilled benzaldehyde, 40 mmol 4-hydroxy-benzaldehyde, 160 mmol freshly distilled pyrrole and 15 ml propionic anhydride. The mixture product was chromatographed on an alumina column and silica column with eluent of dichloromethane and chloroform. The elute was concentrated. The crude product was recrystallized giving purple crystalline 4d with a yield of 10% . 1H-NMR (300MHZ, Acetone-d6, d in ppm): 8.92 (8H, pyrrole), 2.80 (4H, hydroxyl), 7.28~7.31 and 8.04~8.08 (16H, phenyl). IR (KBr tablet, cm-1): 3504.4, 1611.0, 1513.0, 1349.5, 1261.1, 1170.6 and 798.7. UV/VIS (lmax, nm in EtOH): 419.5 (the soret band), 516.0, 556.0, 597.0 and 649.0 (the Q band). Elemental Analysis, found (calculated for C44H30N4O): C 82.69 (82.99), H5.91 (5.70), N 8.76 (8.80). 2 RESULT AND DISCUSSION
The condensation of aldehyde with pyrrole exists an equilibrium of ring-chain formation. So the yields of the porphyrins are usually not very high. People have made some attempts to improve the yield of porphyrin, but there were some problems remain to be solved. The formation of each THPP molecule involves the condensation of four hydroxy-benzaldehydes with four pyrroles. Four water molecules were also formed at the same time. Therefore, based on the equilibrium shift principle, timely removing the produced water will promote the process of the condensation, thus result in a higher yield. With this consideration, we used propionic anhydride as a dewatering agent. Results showed an obvious improvement in the porphyrin yield. Propionic anhydride has a higher boiling temperature than propionic acid, hence may allow the reaction system to reflux at a higher temperature. According to the literature and our own experiences, an appropriate high reaction temperature is beneficial to the formation of porphyrin for the Alder condensation.
In the process of Adler condensation of pyrrole with aldehyde, the hydroxyl groups on porphine ring easily form a hard black solid with acid. Therefore, when condensation is completed, a large amount of alcohol is necessary to be added at once under vigorous stirring. By adding alcohol, the strong interaction between the hydroxyl group on porphine ring and the carboxyl groups coming from propionic acid and anhydride was destroyed. The interaction was believed to take place in a short time and not strong at all. Through the addition of a large amount of alcohol with vigorous stirring, the above interaction will be destroyed and the hydroxyl porphyrin will be liberated, thus much higher purity product will be obtained. Otherwise, the isolation of product will be very difficult.
After the addition of a large amount of alcohol, the reaction mixture was filtered. The selection of an appropriate washing solvent is very important for the purity of the product. Using chloroform as the washing agent did not give sufficient dissolution to the solid impurities, therefore the product was still quite impure. Using 95% alcohol instead, both of the product and impurities were well dissolved, resulting in failing in product isolation. A mixture of alcohol and propionic acid with 1:1 in volume was proved to be the best washing solvent in our experiment. By using this mixture as washing solvent, brilliant blue crystal product was obtained.
For the modified Adler method, the optimum adding amount of anhyride was found to be a molar ratio of 2:3 to 1:1 to pyrrole. As shown in Table 1, with the increase of the anhydride, porphyrin yield was improved gradually while the molar ratio of anhydride to pyrrole was less than 2:3. Over 1:1 of the molar ratio of anhydride to pyrrole, further more amount of anhydride would have no effect on the increase of porphyrin yield.
Table 1 The effect of anhydride addition on the yield of T(4-HP)
Anhydride/pyrrole ( molar ratio ) |
0 |
1/3 |
1.5/3 |
2/3 |
1/1 |
Yield ( % ) |
10.1 |
17.1 |
17.7 |
21.4 |
22.0 |
[1] Li G N, Wu S D, Teng Y W. Chinese Journal of Org. Chem. (You Ji Hua Xue), 1985, 5 (4): 300.
[2] Tong S Y. Chemical Regents (Hua Xue Shi Ji), 1979, 1 (3): 32.
[3] Jon Tokkyo Koho, JP61130220, 1986.
[4] Liu W, Shi T S. Chem. J. Ch. Univ (Gao Deng Xue Xiao Hua Xue Xue Bao), 2001, 22 (1): 16.
[5] Duncan W Bruce, Michael A Wali , Wang Qing Min. J.Chem.Soc.chem.commun,1994: 2089.
[6] Liu W, Tao J Z, Shi T S. Chinese Jaunal of Applied Chemistry (Ying Yong Hua Xue), 2001, 18 (3): 228.
[7] An Q D, Wang D Z, Shi J S et al. Chinese Journal of Organic Chemstry (You Ji Hua Xue), 2001, 21 (8): 606.
[8] Zhao H B, Cai J, Lin Y B et al. Chinesse Jouranl of Organic Chemistry (You Ji Hua Xue), 2000, 20 (3): 424.
[9] Tsuchida E, Keruyuki T, Arai K et al. Chem. Soc. Dalton Trans, 1993, 2465.
[10] Komatsu T, Kumamoto S, Nishide H et al.The Chemical Society of Japan, 1993: 1640.
[11] Adler A D, Longo F D, Finarelli J D et al. J. Org. Chem., 1967, 32 (70): 476.
[12] M Momenteau, J Mispelter, B Loock et al. J. Chem. Soc., Perkin Trans. I, 1983, (1): 189.
[13] Gottwald L K, Ullman E F. Tetrahedron Lett, 1969, (36): 3071.
[14] Little R G, Loach J A, Ibers J A. J. Heterocycl. Chem, 1975, 12 (2): 343.
[15] Cheng D Q, Liu H C. Chemical Reagents (Hua Xue Shi Ji), 1991, 13 (3): 149.
[16] Tong S Y, Zhao F L, Shun G B. Analytical Chemistry (Fen Xi Hua Xue), 1987, 15 (12): 1067.
¡¡