http://www.chemistrymag.org/cji/2002/049042pe.htm |
June 30,
2002 Vol.4 No.9 P.42 Copyright |
Effect of alkaline metals on low temperature activity of the vanadium catalyst
Chen Zhenxing, Huang Qiaoping
(College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Received Mar.28, 2002; Supported by the National Natural Science Foundation of China(20176065).
Abstract For vanadium catalyst that was
prepared with carbonized mother liquor, the effect of alkali-metal elements such as Cs,
Rb, Li on low temperature activity had been investigated. The result shown that Cs was the
best promoter among above alkali-metal elements and the suitable n(Cs)/n(V) varied in the
range of 0.3-0.6. As n(Cs)/n(V)=0.6, the conversion of SO2 would increase to
42.5% at 410oC, which was 18.5% higher than that of catalyst without Cs. Because
of the negative effect on low temperature activity of vanadium catalyst, the content of Rb
existed in carbonized mother liquor should be cut down as little as possible. Li had a
good effect on low temperature activity of catalyst if there existed other kind of
alkali-metals in catalyst, its optimum dosage was n(Li)/n(V)=0.4 and then the conversion
of SO2 was 46% at 410oC. Furthermore, the content of active melting salt had a great
influence on low-temperature activity of catalyst and the suitable n(M)/n(V) was 4.5.
Keywords promoter; alkaline metal; melting salt; low-temperature activity; sulfuric
acid; sulfur dioxide
1 INTRODUCTION
To increase the low-temperature activity of vanadium catalyst, the catalysis of alkaline
metals, lanthanum metals, P2O5, B2O3, MnO2,
W2O3, etc, on vanadium series catalysts had been investigated [1-5].
Among them, the promotional catalysis of alkali metal elements like Cs, Rb, Li, Na and K
had been thoroughly investigated. However, the conclusions were remarkably different. In
some metallurgical factories, there existed carbonized mother liquor (CML), in which
alkali metal elements such as Cs, Rb, Li, Na and K were abundant, but noxious elements
were extremely rare. As vanadium catalyst was prepared with CML, the conversion of sulfur
dioxide reached to 35.6% (mole ratio) at 410oC, which
had reached to the level of national standard (35%)[6-8]. To prepare
low-temperature vanadium catalyst with higher activity, more alkali metal elements like
Cs, Rb and Li was added into the catalyst and its effect on catalytic activity was
investigated.
2.1 Materials
The refined diatomite was provided with a vanadium catalyst plant. CML primarily included alkali-metal carbonate and hydroxyl compounds. Chemical reagent included V2O5, sulfuric acid, alkali metal sulfates and sulfur powder and etc.
2.2 Preparing process of catalyst
The preparation process of vanadium series catalyst was shown in Fig.1. To begin with, vanadium pentoxide, small amount of alkali metal sulfate was mixed proportionally with CML in a tank. After they had been dissolved sufficiently, sulfuric acid was added into the tank in order to neutralize (pH=2~4). Then, diatomite and sulfur powder was added into the tank. After the stuff had been sufficiently kneaded, a ram extruder was adopted for shaping. Finally, through dried, baked and sieved, catalyst samples with the size of 5กม(5~10)mm were obtained.

Fig.1 Preparation of vanadium catalyst
2.3 Testing and analysis
The experimental apparatus used to test catalytic activity was shown in Fig.2. The
concentration of sulfur dioxide was 10% (mole ratio) and air velocity was 3600h-1.
The integral reactor was made of stainless steel and its size was dia30กม2mm,
in which 30ml catalysts was put into the reactor. The temperature was controlled by XT101
type silicon-controlled temperature-controller and reaction temperature was detected
through UJ36 type potentiometer. Iodometry was adopted to analyze the concentration of
sulfur dioxide. The partial pressure of oxygen was determined through CM type oxygen
measurer. The total content of alkali carbonate and alkali hydroxide was measured through
double indicator titration. The content of Cs and Rb was tested by PW1401 type maleinoid
form X-ray diffraction fluorescent spectrometer. The content of Li, Na, K was measured by
PS-6 type inductively coupled plasma atomic emission spectrometer. DuPont 9900 type
thermal analyzer was used to test the differential thermal analysis curve.
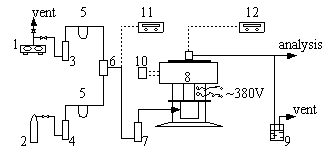
Fig.2 Apparatus for activity test
1-air compressor 2-sulfur dioxide steel bottle 3-silica
gel drying bottle 4-concentrated surfuric acid drying bottle 5-capillary flow gauge 6-gas
mixer 7-molecular sieve drying bottle 8-integral reactor 9-alkali liquor absorption bottle
10-temperature controller 11-CM type oxygen indicator 12-UJ36 type potentiometer
3.1 The effect of the content of Cs on low temperature activity
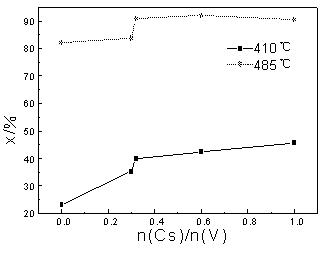
Fig.3 The effect of the content of Cs on catalytic activity
(W(V2O5)=6.5%, n(K)/n(V) =3.0, n(Na)/n(V) =1.0)
For low-temperature vanadium catalyst, in
which CML was used as main promoters, the dosage of CML was properly controlled to make
w(V2O5)=6.5% (mass ratio). As n(K)/n(V)=3.0 (mole ratio) and
n(Na)/n(V)=1.0, catalytic activity was measured with the change of content of Cs and the
results were shown in Fig.3. As n(Cs)/n(V) was increased from 0 to 1.0, the conversion of
sulfur dioxide would be improved from 23.0% to 45.6% at 410oC, from
81.9% to 90.2% at 485oC. But there existed an apparent turning point. As n(Cs)/n(V) was
beyond 0.31, the conversion of sulfur dioxide did not change with the increase of
n(Cs)/n(V) at 485oC to some extent and the
conversion changing curve at 410oC tended to be flat. Therefore,
as far as medial-temperature activity was concerned, 0.31 was the suitable n(Cs)/n(V). For
low-temperature activity, the conversion of sulfur dioxide could be slowly increased as
n(Cs)/n(V) was beyond 0.31. But if the production cost was regarded, n(Cs)/n(V) should be
controlled in the range of 0.3~0.6.
The reason that catalytic activity could be increased prominently under
low temperature was that Cs salt and V pentoxide could combined to form a low co-melting
complex --Cs2S2O7-V2O5, which made
the melting point of catalyst decreased by 30-50oC and then reduced the precipitation of V4+.
3.2 The effect of the content of Rb on low temperature activity
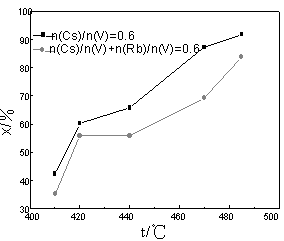
Fig.4 The effect of Cs and Rb on catalytic activity
(W(V2O5)=6.5%, n(K)/n(V) =3.0, n(Na)/n(V) =1.0)
To compare the promotional catalysis of Cs and Rb, Rb salt was added into above Cs
catalyst to make n(Cs)/n(V)=0.3 and n(Rb)/n(V)=0.3, the activity of this kind of catalyst
was shown in Fig.4. As a contrast, the activity of catalyst, in which n(Cs)/n(V) was 0.6,
was also shown. It was clearly seen that under low-temperature and medial-temperature, the
activity of catalyst in which n(Cs)/n(V) was 0.6 was apparently higher than that of
catalyst in which both n(Cs)/n(V) and n(Rb)/n(V) were 0.3. For example, the conversion of
sulfur dioxide of Cs catalyst was 7.2% higher than that of Cs-Rb catalyst at 410oC. The activity of Cs-Rb catalyst at 410oC was 35.1% while that of Cs catalyst was 35.3% (as (Cs)/n(V)=0.3),
so it could be concluded that the addition of Rb would decrease catalytic activity under
low temperature. Consequently, CML should be purified so as to reduce the content of Rb.
3.3 The effect of the content of Li on low temperature activity
As Li was used as promoter of vanadium catalyst, there existed many debates on its
promotional effect [1-5]. Some scientists believed that the promotional effect
of Li was less than that of other alkali metal elements. Some believed Li had no
promotional effect or even a negative effect. But, most of them believed that small radius
cationic Li+ strongly polarized could increase the solubility of VO2+
in the melting salt and prompt melting salt to form vitreous state, and then precipitation
of V4+ could be inhibited and it was good for enhancement of low temperature
activity of V catalyst. Actually, catalytic activity under low temperature was resulted
from common promotional effect of multiple components. Hence, it was necessary to
investigate the promotional effect of Li.
Refined liquor A, which had been obtained by separation of CML, was
blended with other materials (including Li salt) according to n(K)/n(V)=3.0 and
n(Na)/n(V)=1.0 in order to prepare Li catalyst. The effect of Li on activity was shown in
Fig.5. As n(Li)/n(V) was increased from 0 to 0.4, the conversion of sulfur dioxide at 410oC would be increased by 10.2%
and at 485oC by 1.5%. As n(Li)/n(V)=0.4,
the activity under low-temperature and medial-temperature reached maximum, which was 46%
and 91.1% respectively. With the increase of n(Li)/n(V), the activity under
low-temperature and medial-temperature would begin to decline.
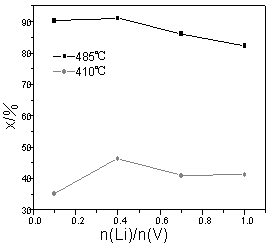
Fig.5 The effect of Li on catalytic activity
(W(V2O5)=6.5%, n(K)/n(V) =3.0, n(Na)/n(V) =1.0)
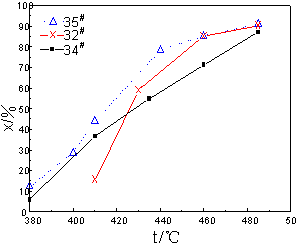
Fig.6 The effect of Li on catalytic activity
(W(V2O5)=6.5%, n(K)/n(V) =3.0, n(Na)/n(V) =1.0)
The activity of catalysts,
which had been prepared with Cs, Rb and Li, was shown in Fig.6. Where, 32# catalyst
was made from V2O5, K carbonate and Na sulfate; 34# catalyst
was prepared with Li salt on the base of 32# catalyst and its n(Li)/n(V)=0.4;
35# catalyst was prepared with Cs salt and Rb salt on the base of 34# catalyst.
It could be concluded that when the reaction temperature was lower than 410oC, the activity of 34# catalyst was higher than that of
32# catalyst. For example, the activity of 34# was twice as high as
that of 32# catalyst at 410oC. However, when the reaction
temperature was beyond 430oC, the activity of 34# catalyst would be lower than that
of 32# catalyst. That indicated that Li salt had a comparably high promoting
effect under low-temperature and it could be used to prepare low-temperature type
catalyst. Whether in low-temperature segment or in medial-temperature segment, the
activity of 35# catalyst was higher than that of 32# catalyst and 34#
catalyst, especially in low-temperature segment. For example, at 410oC the activity of 35#
catalyst would reach to 44%, about 10% higher than that of 34# catalyst. At 485oC the conversion of SO2
on 35# catalyst was also higher than that of 32# catalyst and it
reached to 91%.
Therefore, the addition of Li salt would improve the low-temperature
activity of vanadium catalyst and decrease the medial temperature activity at the same
time. But if Cs salt and Rb salt were properly added into catalyst, Li element would be
added together with other alkali elements, which simultaneously improved the
low-temperature activity and medial-temperature activity
3.4 The effect of the dosage of melting salt on low temperature activity
V2O5 and alkali metal salts were the active components of vanadium
catalyst, which covered on the surface of diatomite under the reaction temperature in a
state of melting salt. The effect of the dosage of melting salt on activity of vanadium
catalyst was shown in Table1. It could be seen that the activity of 26#
catalyst was increased by 3.9% compared with that of 22# catalyst at 410oC, but at 485oC the activity of 26# catalyst was decreased by 17.2%
compared with that of 22# catalyst. In 31# catalyst, the n(M)/n(V)
kept unchangeable, but w(V2O5) was decreased from 6.5% to 5.0%,
which made the content of active components decreased to 31%, accordingly, the conversion
at 485oC was increased to 89%. The
reason that the medial-temperature activity of 26# catalyst declined apparently
was that the melting salt was easy to melt under medial-temperature so that partial pores
of diatomite were choked, which made gaseous molecular diffusion resistance increased.
However, under low temperature it is difficult for the salt to melt, so choking phenomena
would not take place and the gaseous molecular diffusion resistance would be also small.
Therefore, as w(V2O5)=6.5%, n(M)/n(V) should be kept to 4.5 in order
to prevent catalytic activity at medial temperature from decreasing.
Cs had a good promoting catalytic activity under low temperature on vanadium catalyst and n(Cs)/n(V) should be controlled in the range of 0.3~0.6. As n(Cs)/n(V)=0.6, the conversion of sulfur dioxide at 410oC reached to 42.5%, which was 18.5% higher than that of catalyst without Cs;
Rb would decrease the activity of catalysts under low temperature. Measures should be taken to reduce the content of Rb in CML;
Li was also a good promoter under low temperature and the optimal n(Li)/n(V) was 0.4 when alkaline-metals(such as Cs, Rb, K and Na) existed in catalyst, and then the conversion of sulphur dioxide at 410oC would reach to 46%;
The content of active components in the catalyst should be controlled. To vanadium catalyst prepared with CML and w(V2O5)=6.5%, n(M)/n(V) should be controlled to 4.5. REFERENCES
[1] Simonova L G et al. Kinetika i Kataliz, 1991, 32 (3): 605-609.
[2] Alvarez E et al. Catalysis Today, 2000, 59 (3): 417-422.
[3] Dunn J P et al. Catalysis Today, 1999, 51 (2): 301-318.
[4] Adlkofer J et al. Sulphur, 1993, (229): 50กซ52.
[5] Kiselev S V et al. Kinetika i Kataliz, 1991, 31 (6): 1318-1322.
[6] Chen Zhenxing et al. Chinese Journal of Catalysis, 2000, 21 (4): 384-386.
[7] Chen Zhenxing et al. Transactions of Nonferrous Metals Society of China, 2001, 11 (3): 462-465.
[8] Chen Zhenxing et al. Journal of Central South University of Technology. 2000, 31 (2): 127-130. กก