http://www.chemistrymag.org/cji/2002/04b052ne.htm |
Aug. 1,
2002 Vol.4 No.11 P.52 Copyright |
A new chemiluminescence system for determination of Copper(
II)Zhang Xiaoyan, Fan Xiaodong#, Liu
Xiaoli, Du Jianqiang
(Department of Chemical Engineering, Northwest University, Xi'an 710069, China; #Department of Chemical Engineering,
Northwestern Polytechnical University, Xi'an 710072,
China)
Received May.31, 2002.
Abstract This paper reports a new complex
chemiluminescence system, i.e., Luminol-H2O2 catalyzed by CuR22-
complex which is called New Copper Reagent. The detection limit is 6.6×10-8 g
/L Cu(II). The linear dynamic range is 1×10-7~1×10-4g/L.
The relative standard deviation(n=5) for 4×10-5
g/L Cu(II) is 2.7%. Foreign ions, such as Fe(II), Fe(III) and Co(II), interfere when exist
in more than 10-fold ratio(w/w) to Cu(II), and several ions can be tolerated when exist in
higher ratio to Cu(II). The tolerable capacity of
interference ions is relatively large. The precision, accuracy and selectivity of the
method are excellent. The procedure has been satisfactorily applied to determine trace
copper in immunogloblulin, foods and Tabellae Moroxydini Hydrochloride.
Keywords Chemiluminescence, Luminol, Salicylic acid, Copper.
1. INTRODUCTION
In comparison with other kinetic determination methods, chemiluminescence (CL) analysis is
rapid, sensitive and does not require expensive instruments[1]. Some CL methods
for copper determination have been reported, but many metal ions interfere the results.
Yan et al. have reported a determination of copper in the luminol CL
system after ion-chromatography separation of copper, but the
CL system itself has poor selectivity. In recent years, therefore, a search has been made
for a better CL system[2-4].CuR2-2(CuR2-2=
Copper Amino Acid Chelate)has been observed to give chemiluminescence when treated with
hydrogen peroxide in alkaline medium. Several metal ions were found to speed the reaction[1,5].
Cu(II) gives the largest effect. The reaction has now been adopted for the determination
of trace copper.
2. EXPERIMENTAL
2.1 Instrument and
reagents
CL meter, model YHF-1(made in China), including
photomultiplier tube, fused-silica reaction cell and a flat-bed recorder. A pH meter,
model 25 (made in Shanghai , China) was used for pH measurements.
Standard copper solution, 1.0mg/mL, prepared by dissolving the required
weight of analytical grade CuSO4·5H2O in the requisite volume
of redistilled water, and diluted further as required; Luminol (MERCK, Germany) solution,
1×10-2 mol/L, and 2.5×10-4mol/ L luminol working
solution was prepared from it; Salicylic
acid solution, 1×10-3 mol/L; Hydrogen peroxide solution, 0.25 mol/L; Buffer
solution, 0.1M KOH-0.1M H3BO4, was adjusted to pH 10.5-11.5.
2.2 Procedure
Clean and dry the reaction cell, then inject 2.0 ml of 2.5×10-4 mol/L luminol
solution, 1.0ml of 2.5 mol/L hydrogen peroxide through one side-tube, and inject 1.00~2.00ml of CuR22- test solution through the other side-tube, measure the peak height of the
recorded kinetic curve of the CL reaction. Drain the cell through
a side-tube.
3. RESULTS AND DISCUSSION
3.1 The CL reaction
A mixture of luminol solution and hydrogen peroxide in alkaline solution, with CuR22-
as catalyst, emits light, which reaches maximum intensity 3 seconds after injection of the
CuR22- test solution, in a further 1 second the light intensity
falls to 50% of the maximum. Various buffer solutions such as 0.1M KOH-0.1M H3BO3
(pH 10.5-11.5), 0.1M NaHCO3-0.1M Na2CO3 (pH 11.0), 0.1M
NH4Cl-NH3(pH 10.5) were tested, and the first one gave the highest
emission intensity ( Fig.1 ).

Fig.1 Chemiluminescence
dynamic curve
1:Luminol-H2O2 system
2:Luminol-H2O2-Cu (II) system
3:Luminol-H2O2-CuR22- system
[luminol]=2.5×10-4mol/L
[H2O2]=0.25 mol/L
[Cu (II)]=4×10-5 g/L
0.1M KOH-0.1M H3BO3, pH 11.0
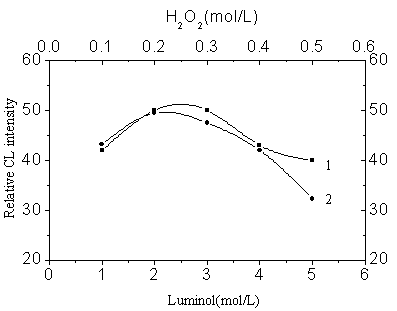
Fig.2 Luminol and peroxide
concentrations
1: Luminol concentration optimization
Initial conditions: [H2O2]=0.20mol/L, [Cu (Ⅱ)]=4×10-5g/L, 0.1M KOH-0.1M H3BO3,
pH 11.0
2: H2O2 concentration optimization
Initial conditions: [luminol]=2.5×10-4mol/L, [Cu(II)]=4×10-5g/L, 0.1M KOH-0.1M H3BO3, pH 11.0; No black signal was detected.
3.2 Optimization
conditions
Fig.2 illustrates the results of the study of optimization of the luminol and peroxide
concentrations. The luminol and peroxide concentrations were 2.5×10-4 mol/L
and 0.20 mol/L respectively. Fig.3 illustrates the effect of the acidity of CuR22-
solution; pH 3 was obtained as optimal.
3.3 Calibration and
detection limit
Fig.4 shows that light intensity is directly proportional to Cu (II) concentration from 1×10-6-1×10-4
g/L. The detection limit is 6.6×10-8 g/L, defined as the concentration
corresponding to the mean background signal plus twice is a standard deviation.
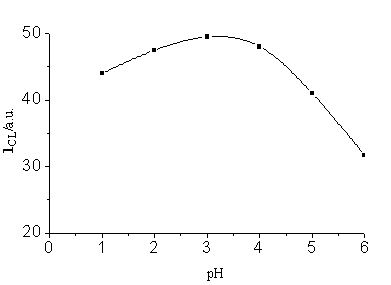
Intial condition: [luminol]=2.5×10-4 mol/L, [H2O2]=0.25 mol/L, [Cu (Ⅱ)]=4×10-5g/L, 0.1M KOH-0.1M H3BO3, pH 11
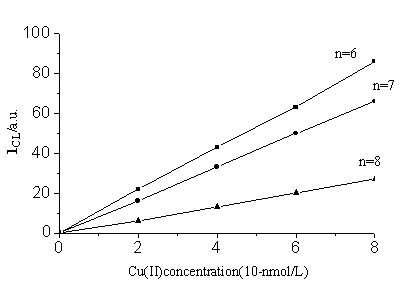
Fig. 4 Calibration curve for Cu (II)
3.4 Interferences
An extensive interference study gave the results shown in Table 1. Although Fe (II), Fe
(III) and Cr (III) interfere the results when exist at
greater than 10-fold ratio (w/w) to Cu (II), they can be screened with 1.0×10-5
mol/L sodium citrate, which does not affect the CL intensity.
Table 1 Influence of coexistent ions
Foreign ions |
Added |
Cu(II)recovered |
Foreign ions |
Added |
Cu(II)recovered |
Al3+ |
1000 |
98.7 |
Mn2+ |
200 |
100.7 |
Ba2+ |
2000 |
100.0 |
Cr (VI) |
200 |
100.1 |
W (VI) |
2000 |
100.0 |
Cr ( III) |
200 |
101.7 |
Mo (VI) |
200 |
99.7 |
B3+ |
1000 |
100.0 |
Mg2+ |
1000 |
100.0 |
Ni2+ |
400 |
101.5 |
Th (IV) |
400 |
100.0 |
Pb2+ |
400 |
100.9 |
Pt (IV) |
200 |
100.0 |
Cd2+ |
400 |
100.3 |
Zn2+ |
200 |
100.0 |
CL- |
1×105 |
100.0 |
Fe3+ |
20 |
112.0 |
SO42- |
1×105 |
100.0 |
Fe2+ |
40 |
116.0 |
NO3- |
1×105 |
100.0 |
3.5 Determination of copper in immunogloblulin, foods and tabelle moroxydini
hydrochloride
The sample is dissolved by heating 0.500g Tabellae Moroxydini Hydrochloride with 5ml of
concentrated nitric acid in a beaker on a controlled hot-plate until nearly dry, then add
1ml of concentrated perchloric acid. It is heated until all white fumes disappeared, and then make up to a volume in a 50ml volumetric
flask. A known volume of this solution is transferred into a 25ml volumetric flask, 1.5ml
salicylic acid is added, pH is adjusted to 3 with nitric acid, and the solution is diluted
to a volume with redistilled water, and analysed as already described. Typical results are
showed in Table 2.
Table 2 Determination of Copper in immunogloblulin, foods and Tabellae Moroxydini Hydrochloride (mean of five determination )
Sample |
Cu (II) found mg/ml | RSD |
Cu added mg/ml |
Cu recovered |
||
CL |
AAS[6] |
mg/ml | % |
|||
200010 |
22.15 |
22.16 |
2.7 |
5.00 |
4.90 |
98 |
200028 |
28.94 |
28.92 |
5.00 |
5.30 |
106 |
|
200036 |
24.75 |
24.75 |
5.00 |
5.05 |
101 |
|
200068 |
21.46 |
21.44 |
5.00 |
5.00 |
100 |
|
REFERENCES
[1] Zhang Xiaoyan, Li Shaoqing.
Chemiluminescence analytical technology. Xi'an Map Publishing House. 2000,189-192.
[2] Yan B, Worsfold P. J. Anal. Chim. Acta. 1990, 236-237.
[3] Zhuang Huisheng, Chen Guonan, Wang Qing'e. Anal. Chem., 2000, 28 (5): 573-576.
[4] Zhang Wuming, Zhong Shiming, Hu Hejin. Analyst, 1989, 17 (10): 922-924.
[5] Li Shaoqing. Chinese Chem. Letter. 1991, 2 (9): 701.
[6] PRC Certified Reference Materials Catalog, State Bureau of Technical Supervision,
1995.