http://www.chemistrymag.org/cji/2002/04b055ne.htm |
Aug. 1, 2002 Vol.4 No.11 P.55 Copyright |
Synthesis of a new ferrous salt of phosphatidic acid
Wang Jian, Liu Ping*, Cheng Jiankai
(Fujian Institute of Research on the Structure of Matter, Chinese Academy
of Science, Fuzhou 350002, China)
Received June.27, 2002; Supported by the National Natural Science Foundation of China and Natural Science Foundation of Fujian Province of China.
Abstract A new compound
1,2-O-Di-octadecyl-sn-glycero-phosphatidic acid ferrous salt hydrate has been synthesized
for first time. Its IR, NMR, Mass spectra and electrical conductivity have been studied.
The novel compound will be studied as a possible second messenger.
Keywords Phosphatidic acid ferrous salts, Synthesis, Spectra.
1 INTRODUCTION
In recent years electrical conduction of some metal complexes has been studied[1,2,3].
And electrical conduction of some ingredients of organism has been attracting much
attention, it is important to investigate electrical conduction and corresponding basic
theory. For example, the study of electrical conduction through DNA molecules has
illustrated that DNA is a semiconductor, but its principle of electrical conduction has
not yet been revealed[4,5].
Phosphatidic acid is one of lipid, although it is only a little in
membrane, but it plays an important role. It is an extracellular messenger in cells. Some
different cells are able to respond to lysophosphatidic acid[6]. In addition,
phosphatidic acid can increase G-protein Rac2-GDI complex dissociation, which shows as
second messenger[8,9]. Phosphatidic acid involves interaction between
extracellular signaling pathways for example, PLC, PLA2, IP3, etc[10,11]. The
sensitivity of PI3-Kinase to PA and lyso-PA could imply cross-talk between the
phospholipase D and PI3-Kinase signal transduction pathways in vivo[12]. But
phosphatidic acids are quite unstable compounds and its use was limited. Salt of
phosphatidic acids is more stable than phosphatidic acid. There are few synthesized sodium
and calcium salt of phosphatidic acid. At some time we note that synthesis and electric
conduction of transition-metal salt of phosphatidic acid are not reported. In this paper,
we have studied a convenient method to synthesize transition-metal salt of phosphatidic
acid taking advantage of substitution reaction from starting material(FeCl2·4H2O)[13].
Its spectra and electrical conductivity were investigated. Its other function and property
will be studied in the future.
2 EXPERIMENTAL
The sodium salt 1,2-O-Di-octadecyl-sn-glycero-phophatidic acid was prepared by known method[14]. Ferrous chloride hydrate(FeCl2·4H2O) was bought from Aldrich Company.

All reactions were performed under an atmosphere of nitrogen.
To a solution of 1,2-O-Di-octadecyl-sn-glycero-phosphetidic acid sodium salt 0.416mmol in CHCl3(9ml), and MeOH(1ml) was added FeCl2·4H2O 0.416mmol and the reaction mixture was stirred at 50¡æ for 20hours, the precipitate was filtrated off and the solvent was put into refrigerator. Light yellow solid 123.0mg was got. Yield of the compound was 37%.
2.1 Element analysis
The element analysis was recorded on a vario ElIII.
2.2 IR spectrum
Infrared spectra of the compound was recorded on a Nicolet Magna 750 Fourier transform infrared spectroscopy respectively. The measurement was made as KBr pellets in the region 4000~400cm-1 and scanned at room temperature for 32 times. And in the region 600~100cm-1 we recorded spectra as CsI pellets for 73 times scanning.
2.3 NMR spectrum
1H-NMR spectra was measured on the Varian Unity-500 superconductivity NMR spectrometer, using TMS as an internal standard, CDCl3 as a solvent.
2.4 Mass spectrum
The molecule-ion mass spectra was measured on a Finnigan MAT-312 GC/MS/DS spectrometer with electron impact(EI) method, at a resolution of 1000. The temperature of the ion source is 160¡æ, the gasification temperature is 250-300¡æ, the accelerating voltage of the ion source is 3KV, the electron energy is 70eV. Ion peaks of a series of mass spectra which could be repeated exactly were collected with the computer.
2.5 Electric conductivity
The powdered sample was compressed into inseparable round plates under 10.5 ton pressure. Electrical resistance was measured at different temperature(from 25¡æ to 80¡æ) with ZL5 model LCR measuring apparatus.
3 RESULTS AND DISCUSSION
3.1 Element analysis
Calcd.(%) for C39H87PO10Fe: C, 46.98; H, 9.43; P, 5.52;
Fe, 9.96.
Found(%): C, 46.87; H, 9.34; P, 5.40; Fe, 9.60.
3.2 IR spectrum
The strong absorption at 1063cm-1 could be attributed to the stretching
vibration of P-O, and the absorption peaks at 2850~2918cm-1 could be assigned
to the stretching vibration of CH3 and CH2, their distortion
vibration should be found in the region 1498~1377cm-1. The broad peak at 3500cm-1
could be attributed to the characteristic absorption of OH in the water crystal. The
middle strong absorption peaks in 422 cm-1 and 390 cm-1 can be
attributed to the stretching vibration of Fe-O[15].
3.3 NMR spectrum
1H NMR(CDCl3/TMS): d0.889(6H,m,CH3),
Table 1 Main Fragments Ions in the Compound
Fragment ion |
m/z |
% |
Fragment ion |
m/z |
% |
C4H9+ |
57 |
100 |
C9H19+ |
127 |
1.61 |
C5H11+ |
71 |
50.98 |
C18H37+ |
253 |
4.55 |
C6H13+ |
85 |
28.9 |
PO4+ |
95 |
3.15 |
C7H15+ |
99 |
6.87 |
Fe+ |
56 |
3.55 |
C8H17+ |
113 |
3.00 |
FeO+ |
72 |
1.97 |
The mass analysis of ferrous salts of phosphatidic acid is carried out for the first time.
Through the detection with computer, main ion fragments and their abundance are listed in Table 1. The ion C4H9+(m/z 57, 100%) is the base peak. See from the above table, we can easily explain the structure of the compound. The serial arithmetical progression molecule ion peaks(C4H9+, m/z 57, 100%; , C5H11+, m/z 71, 50.98%; C6H13+, m/z 85, 28.90%; C7H15+, m/z 99, 6.87%; C8H17+, m/z 113, 3.00%; C9H19+,m/z 127, 1.61%; C18H37+, m/z 254, 4.55%) shows that the octadecyl exists. Besides, we have obtained the fragments of PO4+(m/z 95, 3.15%), which supports the phosphatidic acid structure. The two peaks of Fe(m/z 56, 3.55%) and FeO(m/z 72, 1.97%) show that the compound belongs to the ferrous salts.
3.5 Electrical Conductivity
From the determined resistance, the diameter and thickness of a plate, the conductivity will be calculated.
The conductivity of ferrous of the phosphatidic acid depended on temperatures is shown in Fig.1. The data is 10-4-10-8 S·cm-1 level of grade. The region belongs to the range of conductivities of conventional semiconductors 10-9-102 S·cm-1. At the same time, the conductivities will rise with the temperature increasing, which completely conform to the characteristics of the relationship between the conductivity and temperature of semiconductor. It can be deemed that this series of compounds belong to the category of unconventional semiconductors.
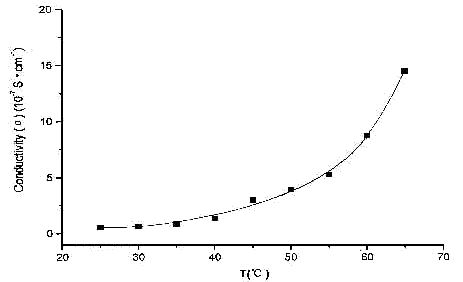
Fig.1 Plots of the Temperature Dependencies of the Conductivities£¨s£©for the Compound
REFERENCES
[1] Papavassiliou G C. Prog. Solid State Chem., 1997, 25: 125-270.
[2] Kahn O, Pei Y, Nakatani K et al. Mol.Cryst.Liq.Cryst., 1989, 176: 481.
[3] Tajima H, Inokuchi A, Kobayashi A et al. Chem.Lett.,1993: 1235.
[4] Fink H W, Schonenberger C. Nature, 1999, 398: 407.
[5] Dandliker P J, Holmlin R E, Barton J K. Science, 1997, 257: 1465.
[6] Kawase T, Suzuki A. J. Biochem., 1998, 103: 581-582.
[7] Pelassy C, Mary D, Aussel C. J. Lipid Medictors, 1991, 4: 199-209.
[8] Heyworth P G, Knaus U G, Xu X et al. Mol. Biol. Cell, 1993, 4: 261-269.
[9] Chuang T H, Bohl B P, Bokoch G M. J. Biol. Chem., 1993, 268: 26206-26211.
[10] Billah M M. Curr. Opin. Immunol, 1993, 5: 114-123.
[11] Exton J H. Biochim. Biophys. Acta, 1994, 1212: 26-42.
[12] Ron Lauener, Yaping Shen, Vincent Duronio et al. Biochem. and Biophys, Res.Commun.,
1995, 250(1): 8-14.
[13] Liu
[14] Paltauf F. Chemistry and Physics of Lipids, 1976, 17: 148-154.
[15] Bellany L. J. Volume II, Second Edition. London and New York. 1980.¡¡
¡¡
¡¡