http://www.chemistrymag.org/cji/2003/056046ne.htm |
Jun. 1, 2003 Vol.5 No.6 P.46 Copyright |
Li Zhiwei1
, Yang Gengliang1,2, Wang Dexian1, Zhou Shengli3, Liu Erdong1, Chen Yi2*(1 College of Chemistry and Environmental Science, Hebei University, Baoding 071002; 2Center for Molecular Science, Institute of Chemistry, Chinese Academy of Science, Beijing 100080; 3Hebei JIHENG Pharmacy Co. Ltd., Hengshui 053000, China)
Received Jan.12, 2003; Supported by the National Natural Science Foundation of China (20075005), the Natural Science Foundation of Hebei Province (202096) and Ministry of Science and Technology.
Abstract A synthetic polymer selector for
aminoantipyrine is prepared by molecular imprinting technology. Methacrylic acid and
ethylene glycol dimethacrylate are copolymerized in the presence of the template
aminoantipyrine. The template is extracted from the polymer leaving specific recognition
sites, complementary to the template. The polymer is utilized as a stationary phase in
HPLC. The mixture of the two close analogues, aminoantipyrine and aminopyrine, can be
baseline separated when the mobile solution is composed of methanol: isopropanol = 2: 8.
When the concentration of isopropanol is 100%, only aminopyrine is eluted and the
aminoantipyrine is completely reserved by the column.
Keywords Molecular Imprinting Stationary Phase, High performance liquid
chromatography, Separation, aminoantipyrine, close analogues.
1 INTRODUCTION
Molecular imprinting polymer (MIP) has drawn
increasing attention in recent years[1-4]. Owning to its higher molecular
recognition ability and stability than that of biological molecules. MIP has been used in
a wide range of applications, such as chiral separartion[5-8], solid phase
extraction[9,10], sensor[11,12], immuno-analysis[13] and
has become an effective strategy to prepare stationary phase of HPLC having a specific
molecular recognition[14].
The first example of molecular imprinting of organic network polymers
introduced by Wulff is based on a covalent attachment strategy, i.e., covalent
monomer-template, covalent polymer-templates[1]. The most successful
non-covalent imprinting systems are based on commodity acrylic or methacrylic monomers,
such as methacrylic acid, crosslinked with ethyleneglycol dimethacrylate. The template
molecule usually involves relatively polar functional groups, such as carboxylic acid,
hydroxyl, amino, and/or aromatic groups, and therefore appropriate host monomers having
functional groups that may interact with the functional groups of the template molecule
through intermolecular interactions such as ionic interaction are often utilized to
compose a more effective imprinted site.
Aminoantipyrine and aminopyrine are the intermediates of analgin which is an alesipyretic and antalgic pharmaceutical.
Aminopyrine had also been used for the treatment of heat exposure and ache, but it is
replaced by analgin because it will arise the deficiency of leucocyte and granular
leudocytes[15].
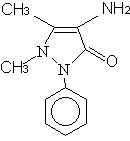
A
B
Fig. 1 The structures of
aminoantipyrine and aminopyrine A=aminoantipyrine, B=aminopyrine
In this study, an aminoantipyrine-imprinted polymer is prepared using aminoantipyrine as the template, methacrylic acid as the functional monomer. Aminopyrine is used as a structurally related reference compound for the evaluation of the selectivity of the imprinted polymer. Furthermore, aminopyrine-imprinted polymer is also prepared. Chromatographic analysis showed that the aminoantipyrine-imprinted polymer is capable of recognizing the functional difference of the two molecules, while the aminopyrine-imprinted polymer have no such ability. The structures of the two molecules are shown in Fig.1.
2. EXPERIMENTAL
2.1 Reagents and chemical
Aminoantipyrine, aminopyrine are provided by Hebei JIHENG
Pharmacy Co. Ltd, (Hengshui, China). Methacrylic acid(MAA) is from The First Tianjing
Chemical Reagent Company(Tinajing, China). Ethylene glycol dimethacrylate (EDMA) is from
Acros(NJ, USA). The compound 2,2'-azobisisobutyronitrile(AIBN) is from the fourth Shanghai
chemical and reagent Factory (Shanghai, China). Acetonitrile is purchased from Fisher
(USA, HPLC grade).
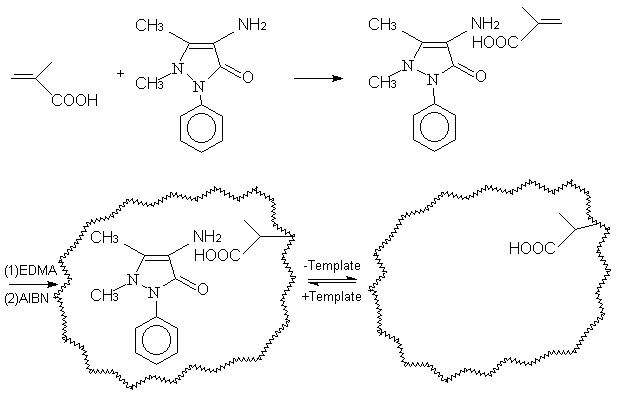
Fig.2 Preparation of imprinted
polymers
2.2 Apparatus
The chromatography system consisted of JASCO (JASCO, Japan) PU-1580 and PU-1586 pumps, an
UV-VIS wavelength UV-1570 detector. Data processing is carried out with a JASCO LC-1500
chromatography workstation. A CS501-SP thermostat is from Sida Experimentation Apparatus
Factory (Chongqing, China). The eluent used for the separation studies is
methanol-isopropyl alcohol. The flow rate is 0.3ml/min, the detector is at 280 nm and the
separation is performed at room temperature.
2.3 Preparation of the stationary phase and packing of the column
Aminoantipyrine-imprinted and aminopyrine-imprinted polymers are prepared identically in
the meantime with MAA as functional monomer. The polymer is synthesized following the
general imprinting protocol shown in Fig.2. 1.0 mmol aminoantipyrine, 6.0 mmol MAA are
dissolved in acetonitrile in a glass polymerization test tube, then 30 mmol EDMA and
0.065g AIBN are added into the solution. The test tube is purged with nitrogen for 10 min
and sealed under vacuum. After that, polymerization is carried out in a water bath with
the temperature maintained at 60ºC for 24 h. The polymerized monolith is ground into particles and by
passing through a 35mm
sieve and by repeated suspending in water to remove the small particles, the size fraction
of 25-35mm is obtained and
it is packed in a 150×4.6mm stainless steel HPLC column. Non-imprinted blank polymers in
the absence of template are prepared and treated in the same manner. Then a solution of
methanol-acetic acid(9:1) is used to remove the template. In the experiments, three
different polymers are prepared, which are the imprinted polymer made up with
aminoantipyrine as the template(P(1)), the imprinted polymer with aminopyrine as the
template( P(2)) and the blank polymer (P(3)).
The separation factor (a) is determined by
a=k'2/k'1,
where k'2 is the capacity factor of the aminoantipyrine and k'1 is
the capacity factor of the aminopyrine.
The capacity factors are determined by
k'= (tR-t0)/t0,
where tR is the retention time of the solute and t0 is void time of
the column, which is determined by NaNO2 as the void marker. All the procedures
are carried out at room temperature.
3 RESULTS AND DISCUSSION
3.1 Effect of templates
Methacrylic acid (MAA) is chosen as the functional monomer because MAA could interact
with the amino groups(see Fig.1) of the compounds. The molecular recognition ability of
the imprinted polymers is evaluated by comparing the retention time of aminoantipyrine and
aminopyrine on the columns packed with the polymers. As shown in Table 1, it can be seen that P(2) and P(3) polymers
had no selectivity for aminoantipyrine and aminopyrine, while the retention and the
selectivity of aminoantipyrine-imprinting polymer P(1) for the two molecules are greatly
strengthened by molecular imprinting. This showed that the amino group in aminoantipyrine
could form hydrogen bond with the monomer and produce specific sites in the polymer.
Aminopyrine, on the other hand, as no amino group exists in the molecular structure and
the amide group could only form so weak hydrogen bond with the monomer that it is hard to
produce effectively specific sites.
3.2 Chromatographic application
P(1) is employed as the stationary phase of the chromatographic column. Different
factors, such as, the composition of the organic solvents, flow rate, temperature, are
investigated to study the influence on the separation of the two analogues.
Table 1 The capacity factors of the analytes on the imprinted polymers
Polymers |
k'1 |
k'2 |
a |
RS |
P (1) |
1.044 |
2.925 |
2.802 |
1.2 |
P (2) |
1.892 |
1.892 |
1 |
- |
P (3) |
1.248 |
1.248 |
1 |
- |


time (min)
time (min)
Fig.4 Chromatograms of the separation of the two analogues. Run
conditions: column,150x4.6mm ; flow rate, 0.3mL/min; wavelength, 280nm; (a)Eluent is 100%
isopropanol (b)Eluent is methanol:isopropanol=2:8.
3.3 Effect of the composition of the organic
solvents
Experiment showed that polarity of the organic solvents had significant effect on the
retention behavior of the template. In Fig.3, it can be seen that the k' value of antipyrine changes slightly, the k' value of aminoantipyrine changes quickly with the change of
concentration of the solvent. On the other hand, it increases with the increase of
isopropyl alcohol concentration in the methanol-isopropyl alcohol system. When the
isopropanol concentration is 100%, only aminopyrine is eluted and the aminoantipyrine is
completely reserved by the column (see Fig.4a).
3.4 Effect of the flow rate and temperature
The migration times of both aminoantipyrine and aminopyrine decrease with the increase of flow rate, but the resolution of the analogues become poor. This may be that the interaction of the temperate molecule with the polymer becomes weak at high flow rate conditions.
Various temperatures changing from 25ºC to 55ºC are also investigated to study the influence on the retention of the two analogues(Fig.5). Under higher temperature, both the template and aminopyrine migrate fast, but the template changes faster than the aminopyrine. Specific interaction of the template and the specific sites showed stronger dependence on temperature.

Fig.3 Effect of the composition of the organic solvents Fig.5 Effect of the different Temperatures
4. CONCLUSIONSFrom the above results, aminoantipyrine imprinted polymer showed better selectivity on the close analogues (Fig.4b). In HPLC application, under given condition, such as, when 100% isopropanol is used as the mobile phase, aminopyrine is not reserved whereas aminoantipyrine is completely reserved. This is very important in separation or purification of the drugs.
REFERENCES
[1] Wulff G, Angew Chen. Int. Ed. Engl., 1995, 34: 1812.
[2] Mayers A G, Mosbach K. Trends Anal. Chem., 1997, 16: 321.
[3] Sellergren B. Trends Anal. Chem., 1997, 16: 310.
[4] Ansell R J, Ramstrom O, Mosbach K. Clin. Chem., 1996, 42: 1506.
[5] Sellergren K, Shea K J. J. Chromatogr., 1995, 690: 29.
[6] Schweitz k, Andersson L L, Nilsson S. Anal. Chem., 1997, 69: 1179.
[7] Hosoya K, Shirasu Y, Kimata K et al. Anal.Chem., 1998, 70: 943.
[8] Schweitz K, Spegel P, NilssonS. Analyst., 2000, 125: 1899.
[9] Andersson L I. Analyst., 2000, 125: 1515.
[10] Sergeyeva T A, Matuschewski H, Piletsky S A et al. J. Chromatogr., 2001, 907: 89.
[11] Kriz O, Ramstrom O, Mosbach K. Anal. Chem., 1997, 69: 345A.
[12] Peng H, Liang C D, He D L et al. Analytical Letters., 2000, 33: 793.
[13] Idziak I, Benrebouh A. Analyst., 2000, 125: 1415.
[14] Sellergren B, Ekberg B, Mosbach K. J.Chromatogr., 1985, 347: 1.
[15] Zhang W S, Li W A. Medicinal Chemistry (Yao Wu Hua Xue), Beijing, China, Higher
Education Press, 1999, 360.
李志伟1 杨更亮 1,2 王德先1 周胜利3 刘二东1 陈义2
(1 河北大学化学与环境科学学院, 保定 071002; 2中国科学院化学所分子科学中心, 北京 100080; 3河北冀衡药业有限公司., 衡水 053000)
摘要 本文以甲基丙烯酸为功能单体,乙二醇二甲基丙烯酸酯为交联剂,合成了一种氨基安替比林分子烙印聚合物。将所得聚合物用作高效液相色谱固定相,研究了对结构类似物氨基安替比林和氨基比林的拆分能力。分析结果表明,在以甲醇:异丙醇=2:8条件下两种结构类似物可以得到基线分离,实验还发现在以100%异丙醇为流动相时只有氨基比林被洗脱出来,而模板分子氨基安替比林完全被保留在色谱柱上。
关键词 分子烙印固定相,高效液相色谱,分离,结构类似物。