http://www.chemistrymag.org/cji/2003/056048pe.htm |
Jun. 1, 2003 Vol.5 No.6 P.48 Copyright |
(College of Physics and Information Technology, Department of Food Engineering, Shaanxi Normal University, Xi'an 710062, China) Received Feb. 24, 2003; Supported financially by the National Natural Science Foundation of China (No.20272035) and the Foundation for University Key Teachers by the Ministry of Education of China (No.2000-65).
Abstract The effects of oleic acid
(OA) and cholesterol (Chol) on the structures of
1,2-Dielaidoyl-glycero-3-phosphoethanolamine (DEPE) dispersions have been investigated by
small angle X-ray scattering. The effect of the addition of OA to DEPE dispersions was
determined. At 0.5 mol% OA, it induces the formation of Im3m (Q229) phase. Less
than 1.0 mol% OA, DEPE-OA mixed films were in Im3m (Q229) phase. At 1.0 mol%
OA, a phase transition from Im3m (Q229) to Pn3m (Q224) occurred, and
above 1.0 mol% OA, DEPE-OA mixed films was in Pn3m (Q224) phase. The effect of
the addition of cholesterol on DEPE mixed films has also been investigated. The
cholesterol promotes the formation of a coexisting Pn3m (Q224) and HII
phases. In certain conditions, the cholesterol promotes the formation of a single Pn3m (Q224)
phase in the DEPE-OA-Chol mixed films. The results indicate that the cholesterol promotes
the formation of the cubic phase more stabler than the inverted hexagonal phase.
Keywords Cubic phase; Phosphatidylethanolamine; Small angle X-ray scattering
Phosphatidylethanolamines (PE) are one of the major building blocks of biological membranes. The structures of the phosphatidylethanolamine in the liquid crystalline state play an important role in the function of biomembrane [1-4]. Hxagonal structure has been reported mainly for phospholipids with unsaturated chain [5,6]. It has been demonstrated that, under suitable conditions of moisture, PE indeed spontaneously forms hexagonal structure [7,8]. The structures and phase transitions of the phosphatidylethanolamine dispersions have been being interesting subjects for understanding the mechanisms of the lipid polymorphism [9,10].
The phosphatidylethanolamine dispersions are often as relatively simple model systems to study properties of biological membrane [11,12]. Recently, non-double layer structures such as several kinds of cubic phases and inverted hexagonal (HII) phase have attracted much attention both in biological and physicochemical aspects. The non-double layer structures have been postulated to play several important biological roles in membrane fusion, a control of the functions of membrane proteins, ultrastructural organizations inside cells, and crystallization of membranous protein [13-15]. De Oliveira et al [16] has found that the1,2-dioleyl-glycero-3-Phosphatidyl-ethanolamine (DOPE) liposomes generally adopt a hexagonal phase. The presence of both oligonucleotide and oleic acid (OA) allowed the system to organize in a lamellar phase below the solid/liquid transition. The oligonucleotide preferably interacted with DOPE liposomes in a hexagonal phase and led OA to be separated out. Sun et al [17] have investigated the effects of eighteen chemical materials of five classes on the structure of biomembrane in the liquid crystalline state. The results show that biomembrane is always in a double layer structure of the liquid crystalline state under the normal physiological condition, but it may turn from the double layer phases into the non-double layer phases if influenced by chemical materials. These non-double layer phases include both the ordinary cubic structure and hexagonal structure besides some complex middle phase. Three new liquid crystalline phases have been discovered for the first time. This review concentrates on physicochemical aspects of the hexagonal (HII) phase and non-lamellar phase transitions with regard to biological lipid system. The topic of cubic phase is also very important and fascinating. These newly discovered structures might be of help to clarifying the physiological function and the disease of grease in the membrane and the transformation from each other.
In this paper, we found that, at 0.5 mol% OA, it induces spontaneous formation of Im3m (Q229) phase. Less than 1.0 mol% OA, DEPE-PVP-OA mixed films were in Im3m (Q229) phase. At 1.0 mol% OA, a phase transition from Im3m (Q229) to Pn3m (Q224) occurred, and above 1.0 mol% OA, DEPE-OA mixed films was in Pn3m (Q224) phase. The cholesterol promotes the formation of a coexisting Pn3m (Q224) and HII phases. In certain conditions, the cholesterol promotes the formation of a single Pn3m (Q224) phase in the DEPE-OA-Chol mixed films. These results will be significant to the research of the physiology and pathology of membrane, to the health of mankind in treating various kinds of disease and to the instruction in health protection and prevention of chemical poisoning, and so on. 2 MATERIALS AND METHODS
2.1 Materials
1,2-Dielaidoyl-glycero-3-Phosphatidyl-ethanolamine (DEPE), cholesterol (Chol), oleic acid (OA) ethylenediamine-tetra-acetic acid (EDTA) and Tris-(hydroxymethyl)-aminome thane (Tris) were purchased from Sigma (USA), poly-vinyl-pyrrolidone (PVP), and other chemical reagents were purchased from Chinese Reagents Corporation.
2.2 Sample preparation
DEPE and OA were mixed in proportion with the desired molar fractions (0.5, 1, 1.5, 2, 2.5, 5 mol% OA, respectively). Then, a fixed amount (15-mol%) of cholesterol was mixed with DEPE and DEPE-OA to form mixed films respectively. The resulting mixtures were dissolved in an organic solvent: chloroform: methanol (3/1 V/V). The mixtures were evaporated to dryness under reduced pressure. The dry mixtures were hydrated at 30ºC with 50 mM Tris containing 5mM EDTA, 100 mM NaCl and 1 mM sodium azide (pH 7.4). When needed, an appropriate amount of PVP was added respectively with the PVP/DEPE ratio of 0.5 wt% to 0.05 wt%. Liposomes were then made by successive extrusion through 0.4 and 0.2 mm polycarbonate filter, except for pure DEPE films that were simply hydrated at 4ºC, below the lamellar to hexagonal phase transition temperature. The samples concentration of the X-ray sample were 5-10 mg/100 mL. The samples were then lyophilized. Aliquots containing 2-4 mg of DEPE were introduced in pre-weighed DSC aluminium pans and hydrate over saturated solution of Mg(NO3)2, at 4ºC (60% relative humidity), until constant weight. The water weight fraction for the different samples ranged between 0.065 and 0.095.
2.3 Small angle X-ray scattering (SAXS) experiments
All SAXS measurements were performed on a Rigaku D/max-rB rotating anode generator, which allows recording of diffraction data in small-angle region. Ni-filtered CuKa -radiation (l= 0.15405 nm) originating from a Rigaku D/max-rB X-ray generator with a rotating Cu-anode operating at 40 kV and 100 mA was used. The 1st and 2nd slit was adjusted to 0.08 mm and 0.06 mm respectively. The 3rd slit was adjusted to the optimum condition. The received and shelter scattered slit was adjusted to 0.1 mm and 0.25 mm respectively. The X-ray path length through the sample was ca. 1 mm. The sample holder was equipped with two thin film mica windows of film well thickness 0.01 mm. Smoothing algorithms were applied to the data.
2.4 Structural Characterization
2.4.1 Lamellar Liquid Crystalline Phase The parameters of the phases are calculated from d, the X-ray repeat spacing of the phase. The long-range translation ordering of lipid/water aggregate (bilayers, cylinders, micelles, etc.) onto one-, two-, or three-dimensional lattices giving rise to Bragg reflections whose reciprocal spacings (Shkl = 1/dhkl) are in the ratios. For example, Lamellar, Sl = l/d, ratios 1, 2, 3, 4, ¡ the lipid bilayer thickness is db = Fdlam. The water layer thickness is dw =dlam - db. The volume fraction of the non-aqueous part of the sample is F= vl /(vl+ vw), where vl is the volume of one phospholipid molecule and vw is the volume of water per phospholipid molecule.
2.4.2 Hexagonal Liquid Crystalline Phase Hexagonal, Shk = 2(h2+h2+hk)½/a
2.4.3 Cubic Liquid Crystalline Phase Cubic, Shkl = (h2 + k2 +l2 )½/a, ratios 1,
2.4.4 Bicontinuous Cubic Liquid Crystalline Phase A body-center cubic phase only gives reflections for which h + k + l = 2n. Bicontinuous cubic phases have been found: the space groups Pn3m (Q224) and Im3m (Q229). For Pn3m the sequence of allowed reflections is hkl = 110, 111, 200, 211, 220, 221/300, 310, ¡¡ relative peak positions:
When only the first three reflection were detected, a cubic phase whose
3.1 Effect of OA on cubic phase formation of DEPE-OA mixture films
DEPE-OA mixed films in the range of 0-2.5 OA/DEPE molar ratio had been examined. With the addition of OA at a molar ratio of 1.5, the X-ray spacing were found in the ratios of 1,
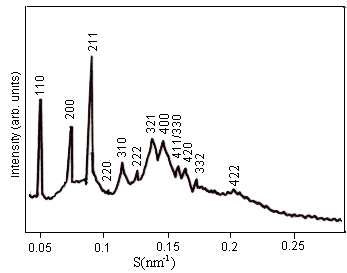
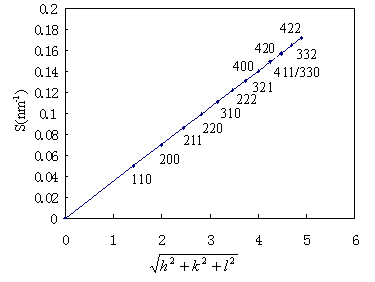
Fig. 1 Effect of 0.5 mol% OA on the DEPE films. The reflections on a cubic phase of space group Im3m (Q229) have disappeared from the X-ray pattern. The lattice constant of this Im3m Q229 phase was 28.5 nm.
We have also investigated the effects of concentration of oleic acid (OA) in DEPE films on their structures at 20ºC by the SAXS method. Addition of small amounts of OA into the DEPE films changed their cubic structure. In Fig 1, the reciprocal d spacing of the remaining small-angle scattering reflections are in the ratio:
We found that, less than 1.0 mol% OA, DEPE-OA mixed films were in Im3m (Q229) phase. As increasing OA concentration in the DEPE-OA mixed films, at 1.0 mol% OA, a phase transition from Im3m (Q229) to Pn3m (Q224) occurred, and above 1.0 mol% OA, the DEPE-OA mixed films was in Pn3m (Q224) phase, with a lattice constant 22.3 nm (Fig. 2(a)). In Fig. 2(b), the pattern is not consistent with a single cubic phase. By analyzing to the Im3m/Pn3m cubic phase mixtures observed in certain conditions in the presence of solutes, the lattice constant of the mixed films gradually decreased with an increase in concentrations of OA. The Im3m and Pn3m lattice parameters were determined respectively. Their ratio is equal to 1.28. An addition of oleic acid changed the appearance of the cubic phase.
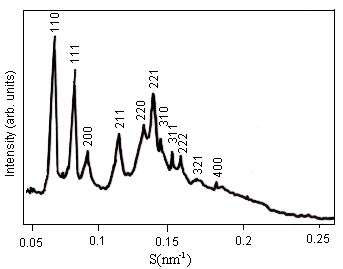
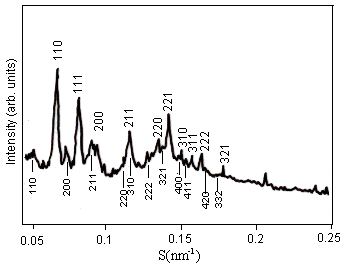
(a) X-ray pattern of Pn3m (Q224) (b) X-ray pattern of Pn3m/Im3m mixtures
Fig. 2 Effect of increasing amounts of OA on the structures of DEPE films was determined by X-ray diffraction. (a) Less than 1.0 mol% OA, DEPE-OA mixed films were in Pn3m (Q224) phase. (b) At 1.0 mol% OA, a phase transition from Pn3m (Q224) to Im3m (Q229) occurred, the two coexisting cubic phases were obtained.
3.2 Effect of Cholesterol on cubic
phase formation of DEPE-OA mixed films
The addition of cholesterol on DEPE-OA mixed films were determined using a fixed added
amount (15 mol % Chol). The X-ray patterns of DEPE-OA-Chol mixed films showed reflections
in the ratios of ![]() :
: ![]() :
: ![]() :
:
![]() :
: ![]() :
: ![]() indicating the presence of
a cubic Pn3m (Q224) phase.
indicating the presence of
a cubic Pn3m (Q224) phase.
At 0.5 wt% PVP, the addition of 15 mol% cholesterol to DEPE-Chol
mixed films can promote the formation of the existence of both the cubic phase and the
inverted hexagonal phase (HII). Both a cubic Pn3m (Q224) phase and a
hexagonal HII phase were found in DEPE-Chol mixed films. The small angle
reflections were reciprocal d spacing in the ratios of ![]() :
: ![]() :
: ![]() :
: ![]() :
:
![]() :
: ![]() :
:![]() and 1:
and 1:![]() : 2:
: 2:![]() .
A nonlamellar phase transition from a cubic phase with lattice constant 22.69 nm (space
groups Pn3m/Pn3) into an inverted hexagonal (H) phase with lattice constant 7.5 nm was
observed. The cubic morphologies probably mediate the transformation from a nonlamellar
phase to an inverted hexagonal phase. The nonlamellar phase transition is as follows: Q(Pn3m)
¡úHII (P6m).
.
A nonlamellar phase transition from a cubic phase with lattice constant 22.69 nm (space
groups Pn3m/Pn3) into an inverted hexagonal (H) phase with lattice constant 7.5 nm was
observed. The cubic morphologies probably mediate the transformation from a nonlamellar
phase to an inverted hexagonal phase. The nonlamellar phase transition is as follows: Q(Pn3m)
¡úHII (P6m).
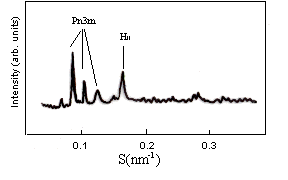
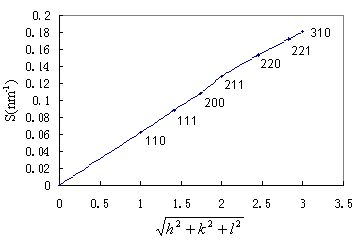
Fig. 3 At 0.5 wt% PVP, the addition of 15 mol% cholesterol to DEPE-Chol
mixed films can promote formation of the existence of both the cubic phase and the
inverted hexagonal phase (HII). Both a cubic Pn3m (Q224) phase and a
hexagonal HII phase were found in DEPE-Chol mixed films. Indexing of the X-ray
diffraction pattern of DEPE-Chol-PVP mixture system as cubic aspect, space group is Pn3m
(Q224). The lattice parameter obtained as the reciprocal slope is a= 22.69 nm.
When a low PVP
concentration (0.05 wt% PVP) in the sample (DEPE-Chol-PVP) was contained, reflection
d-spacing at 16.684, 13.631, 11.928, 10.384, 8.249, 7.870, 7.544, 7.005, 6.396, 5.887, and
5.644 nm are observed with the reciprocal d-spacing in the ratios of ![]() :
:![]() :
: ![]() :
: ![]() :
:
![]() :
:![]() :
: ![]() :
: ![]() :
: ![]() :
:
![]() :
: ![]() . These X-ray patterns suggested the cubic phase with lattice constant
23.59 nm (space groups Pn3m).
. These X-ray patterns suggested the cubic phase with lattice constant
23.59 nm (space groups Pn3m).
We have also examined the effect of cholesterol
on the phase behaviour of a DEPE-OA-CHOL system (84:1:15 molar ratio). At the presence of
both a high and low PVP concentration (0.5, 0.05 wt% respectively), the cholesterol
promote the formation of a Pn3m (Q224) phase. At the X-ray patterns of
DEPE-OA-CHOL mixed films, reflection d-spacing at 14.236, 11.488, 10.068, 8.249, 7.005,
6.715, 6.396, and 5.844 nm, are observable, with the reciprocal d-spacing in the ratios of
![]() :
:![]() :
: ![]() :
: ![]() :
: ![]() :
:
![]() :
:![]() :
: ![]() , characteristic for the
cubic phase of cubic aspects #6 (single space groups Pn3m/Pn3) with lattice constant 20.13
nm. In the DEPE-OA-Chol mixed films, the Pn3m (Q224) phase was found at both a
higher and lower PVP concentration. These results indicate that the cholesterol promote
the formation of a cubic phase more stabler than the inverted hexagonal phase.
, characteristic for the
cubic phase of cubic aspects #6 (single space groups Pn3m/Pn3) with lattice constant 20.13
nm. In the DEPE-OA-Chol mixed films, the Pn3m (Q224) phase was found at both a
higher and lower PVP concentration. These results indicate that the cholesterol promote
the formation of a cubic phase more stabler than the inverted hexagonal phase.
The extreme condition required to obtain stable PE dispersions are unacceptable for most applications. Stable dispersions can, however, be formed under physiological conditions by the addition of a second compound to PE, including fatty acids [18], cholesterol [19], and so on. Oleic acid (OA) can also stabilize PE bilayers under physiological condition [20]. Koynova et al [21] found, with increasing fatty acid content, the nonlamellar phases are arranged in this sequence: bicontinuous cubic phases (Im3m, Pn3m, and Ia3d)® hexagonal phase (H) ® micellar cubic phase (Fd3m)® isotropic phase (I). At higher fatty acid content (85 mol%), micellar cubic phase of space group Fd3m is formed in the two phosphatidylcholine (PC)/fatty acid (FA) mixture. At lower FA content (75-mol%) at least three cubic phases (Im3m, Pn3m, and Ia3d) are formed. We found that the chemical materials can make the biomembrane in the liquid crystalline state assume non-double layer structure [17]. Cholesterol, unsaturated fatty acid and non-ion activated agent have an obvious effect on the liquid crystal structure of phosphatidylethanolamine liposomes [23]. The presence of OA intruded the DEPE mixed films to form a coexisting cubic phase. Less than 1.0 mol% OA, DEPE-OA mixed films were in Im3m (Q229) phase. At 1.0 mol% OA, a phase transition from Im3m (Q229) to Pn3m (Q224) occurred, and above 1.0 mol% OA, DEPE-OA mixed films was in Pn3m (Q224) phase. The cholesterol promotes the formation of a coexisting Pn3m (Q224) and HII phases. In certain conditions, the cholesterol promotes the formation of a single Pn3m (Q224) phase in the DEPE-OA-Chol mixed films. Because cholesterol merely possesses a 3-OH as a polar head-group, it only begins to stabilize the L phase of PE when included at greater than 30-mol%. These results indicate that the Pn3m (Q224) phase is more stable than the Im3m (Q229) phase in the DEPE films, and that the cholesterol promotes the formation of the cubic phase more stabler than the inverted hexagonal phase.
It is also interesting to note that these variations were tightly coupled in such a way that the lattice parameter ratio of the coexisting Im3m and Pn3m phases always remained equal to 1.28. It is consistent with the 1.28 Im3m/Pn3m lattice parameter ratio, as can readily be seen by comparing the specific areas of the Im3m and Pn3m unit cells (28.5 and 22.3). This lattice parameter ratio in the latter range remains very close to the theoretical value. Because, in an infinite periodic minimal surfaces (IPMS) representation, the Pn3m, Im3m, and Ia3d phases correspond to the diamond (D), primitive (P), and gyred (g) surfaces, respectively [22]. The unit cell dimensions of these phases in the states of cubic-cubic coexistence could be expected to satisfy the ratio D: P: G=1:1.28:1.58. It is interesting to point out that the Im3m and Pn3m phases induced in DEPE mixed films appear to comply with such an IPMS representation. In the case of amphiphile/water, Ia3d requires the least amount of water, Pn3m requires more water, and finally Im3m requires the most. These accounts for the observation that the Im3m phases is usually formed at a higher water concentration than the Pn3m phase for various lipid/water systems. Moreover experimentally, by changing the chemical composition of the amphiphile/water system (e.g., adding more water), the Pn3m structure can be induced to go into Im3m merely through a twist of the surface, without any breakage and reassembling of aggregate molecules. In the process, the change in Pn3m to Im3m must necessarily pass through the Ia3d phases. If not, the change in Pn3m to Im3m will not pass through the Ia3d phase. In our experiment, the Ia3d phase was not found from changing Pn3m into Im3m. The Im3m structure can be induced to go into Pn3m were suggested by changing the chemical composition of the DEPE mixed system (e.g., adding OA and Cholesterol). In the process, the change in Im3m to Pn3m not only may be through a twist of the surface, but may be through the breakage and reassembling of aggregate molecules as well.
REFERENCES
[1] Seddon J M, Cevc G, Kaye R D et al. Biochemistry, 1984, 23: 2634.
[2] Sun R G, Zhang J, Qi H. Acta Chimica Sinica (Huaxue Xuebao), 2002, 60 (5): 841.
[3] Zhang J, Sun R G. Chinese Physics, 2002, 11 (8): 776.
[4] Sun R G, Qi H, Zhang J. Acta Physica Sinica (Wuli Xuebao), 2002,51 (6); 1203.
[5] Cullis P R, De Kruijff B. Biochim. Biophys. Acta, 1979, 559: 399.
[6] Sun R G, Zhang J. Acta Biophysica Sinica (Shengwuwuli Xuebao), 2001, 17 (1): 53.
[7] Seddon J M. Biochim. Biophys. Acta, 1990, 1031: 1.
[8] Harlos K, Eibl H. Biochemistry, 1981, 20: 2888.
[9] Litzinger D C, Huang L. Biochim. Biophys. Acta, 1992, 1113: 201.
[10] Seigel D P. Biophys. J., 1999, 76: 291.
[11] Blume G, Cevc G. Biochim. Biophys. Acta, 1990, 1029: 91.
[12] Allen T M, Hong K, Papahadjopoulos D. Biochemistry, 1990, 29: 2976.
[13] Luzzati V, Delacroix H, Gulik A. J. Phys. II France, 1996, 6: 405.
[14]Zhang J, Sun R G, Qi H et al. Acta Chimica Sinica (Huaxue Xuebao), 2000, 56 (6): 704.
[15] Sun R G, Qi H, Zhang J. Acta Photonica Sinca (Guangzi Xuebao), 2002, 31 (11): 1344.
[16] De Oliveira M C, Fattal E, Couvreur P et al. Biochim. Biophys. Acta, 1998, 1372: 301.
[17] Sun R G, Zhang J, Wang Y C. Science in China (Series B), 1998, 41(1): 1.
[18] Duzgunes N, Straubinger R M, Baldwin P A et al. Biochemistry, 1985, 24: 3091.
[19] Epand R M, Bottega R. Biochemistry, 1987, 26: 1820.
[20] Straubinger R M, Duzgunes N, Papahadjopoulos D. The Federation of European Biochemical Societies (FEBS ) Letters,
1985, 179: 148.
[21] Koynova R, Tebchov B, Rapp G. Colloids and Surfaces a Physicochemical and Engineering
Aspects, 1999, 149: 571.
[22] Benedicto A D, O'Brien D F. Macromolecules, 1997, 30: 3395.
[23] Sun R G, Zhang J, Wang Y C. Acta Biophysica Sinica (Shengwu Wuli Xuebao), 1999, 15
(4): 675.