http://www.chemistrymag.org/cji/1999/011001pe.htm |
|
Mu Shaolin , Liu Jincui
(Department of Chemistry, School of Sciences, Yangzhou University, Shouxi Lake Campus,
Jiangsu, 225002, China)
Received
May 21, 1999; Supported by the National Natural Science Foundation of China.(Grant No. 29673036)Abstract Studies on the
electrochemical activity and electrochromism of polyaniline were carried out in NaCl
solution, phosphate solution and solution consisting of ZnCl2 and NH4Cl
using cyclic voltammetry, charge and discharge at a constant current and in situ
spectroelectrochemical techniques. In 1 mol dm-3 NaCl solution with pH 4.57,
there is no redox peaks on the cyclic voltammograms, so polyaniline has a very low
electrochemical activity and the color of polyaniline film changes hardly with potential.
In 0.2 mol dm-3 phosphate buffers at pH 4.57, 6.40 and 7.38, there are redox
peaks on each cyclic voltammogram. Their peak potentials shift toward negative potentials
with increasing pH value. Based on the area of the cyclic voltammogram, the
electrochemical activity, i.e, the redox charge of polyaniline in 0.2 mol dm-3 phosphate
buffer is about three times larger than that of in 1 mol dm-3 NaCl
solution at the same pH value. In 0.2 mol dm-3 phosphate buffer, the starting
potential for the shift of the wavelength of the absorption peak is 0.10 V(vs.SCE) at pH
7.38, 0.30 V at pH 6.40 and 0.40 V at pH 4.57. The tendency of this potential shift is
similar to that of the redox peaks on the cyclic voltammograms. An isosbestic point as a
function of the potential is observed in the plot of the visible spectra of polyaniline in
0.2 mol dm-3 phosphate buffers with pH 6.40 and 4.57. The change in the color
of the film from reddish purple to blue, green and transparent yellow was observed as the
potential decreases from 0.60 to -0.20 V(vs. Ag/AgCl with a saturated KCl solution) in 0.2
mol dm-3 phosphate buffer at pH 4.75 and pH 6.40. In the solution consisting of
2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl,
there are two pairs of redox peaks on the cyclic voltammograms at pH 4.40, pH 4.57 and pH
4.92; their peak potentials shift toward the negative potentials with increasing pH value.
However, the electrochemical activity, and the charge and discharge capacities of
polyaniline at pH 4.92 is larger than that at pH 4.40.
Keywords Polyaniline, Buffer solutions, Electrochemical activity, Charge-discharge
capacity, Electrochromism
1. INTRODUCTION
Polyaniline has excellent electrochemical reversibility and stability in air
and aqueous medium. The color of polyaniline film in aqueous solution can change very
quickly with applied potential. Therefore, polyaniline can be used for batteries electrode[1-3],electrochromic
devices[4,5], light-emitting diodes[6] and immobilization of enzymes[7,8].
The electrochemical properties[9-15] and electrochromism[16,17] of
polyaniline have been extensively studied. However, most of the studies were carried out
in solutions of strong acids with pH values less than 4.0, because of the decrease in the
electrochemical activity and the degeneration of electrochromism in the solutions with pH
values higher than 4.0. A great disadvantage for the solutions with lower pH values is
that the zinc counter electrode in polyaniline batteries is corroded more easily, the
activity of the enzyme is destroyed , as well as the conducting glass used in the
electrochromic devices of polyaniline is easily damaged.
The reduction process of polyaniline is very sensitive to the proton
concentration, because polyaniline must accept protons from the solution . In solutions of
high pH value, there are not enough protons to supply the needs of the reduction of
polyaniline. As a result, polyaniline can not be reduced completely, which affects the
electrochemical activity of polyaniline. It is well known that, a buffer solution has a
large buffer capacity, so the situation for the redox of polyaniline may be changed in the
buffer solutions. Therefore we employ the buffer solutions to study the electrochemical
activity and electrochromism of polyaniline. In this paper, we report the effects of the
buffer solutions on the electrochemical activity, electric capacity during charging and
discharging processes and electrochromism of polyaniline.
2. EXPERIMENTAL
The chemicals used were all reagent grade. Aniline was distilled before use. The
electrolysis cell consisted of a platinum or indium-tin oxide(ITO) working electrode, a
platinum counter electrode and a reference electrode of the saturated calomel
electrode(SCE).
A PAR Model 173 potentiostat-galvanostat with a Model 179 digital
coulometer was used for the electrochemical polymerization of aniline. The potential was
set at 0.80 V(vs.SCE) for the electrolysis of aniline. A HPD-1A potentiostat-galvanostat
was used for the cyclic voltammetry of polyaniline films. The scan rate was 50 mV s-1.
Their cyclic voltammograms were recorded using a YEW Model 3036 X-Y recorder. The
instruments used for the charge and discharge of the polyaniline battery were an automatic
battery charge-discharge unit(HJ-201B) and a YEW 3066 pen recorder. The measurements of
the UV-visible spectra of polyaniline film polymerized on an ITO conducting glass were
carried out on a Beckman model Du-7u spectrophotometer. The temperature for the
electrochemical experiments was controlled at 20oC.
3. RESULTS AND DISCUSSION
3.1 Effect of the buffer solutions on the electrochemical activity
Polyaniline for this experiment was polymerized on a platinum foil(4×4
mm2). The total charge during the electrolysis of aniline was 0.024 C. The cell
for the cyclic voltammetry consisted of a polyaniline electrode, a platinum counter
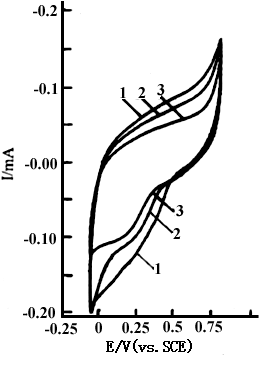
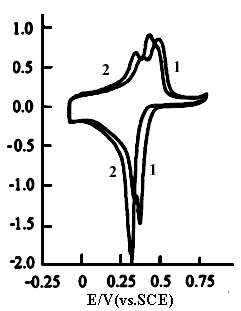
electrode and an SCE. Curves 1,2 and 3 in Fig.1 show the cyclic voltammograms of
polyaniline film in 1 mol dm-3 NaCl solution at pH 4.57,6.40 and 7.38,
respectively. The sweep potential was between -0.10 and 0.80 V(vs.SCE). There are no
oxidation and reduction peaks in Fig.1, but their redox currents decrease with increasing
pH value. This means that the electrochemical activity of polyaniline decreases with
increasing pH value.
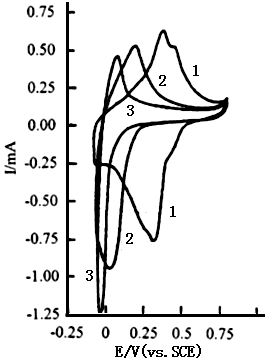
Curves 1,2 and 3 in Fig.2 show the cyclic voltammograms of polyaniline
in 0.2 mol dm-3 sodium phosphate buffer at pH 4.57,6.40 and 7.38, respectively.
It is clear that there are two oxidation peaks and a reduction peak with a small shoulder
on curve 1, and an oxidation peak and a reduction peak on curves 2 and 3. From the areas
of cyclic voltammograms of each curve in both Fig.1 and Fig.2, one can see that the
electrochemical activity of polyaniline, i.e., the redox charge of polyaniline in 0.2 mol
dm-3 phosphate buffer is about three times larger than that in 1 mol dm-3
NaCl solution at each pH value. Figure 2 shows that the potentials of the anodic peak and
the cathodic peak shift toward the negative potentials with increasing pH value, which
leads to the decrease in the redox charge of polyaniline. The shifts of the redox peaks
are analogous to those of polyaniline in HCl solutions with pH < 4 [9].
However, no shift of the peak potential with pH is observed in Fig.1. This indicates that
the shape of the cyclic voltammograms and the electrochemical activity of polyaniline are
affected markedly by the solution composition. It is well known that the phosphate buffer
has a large buffer capacity, so it can immediately supply enough protons for the reduction
of polyaniline during the negative potential scan. As a result, polyaniline in the
phosphate bufferis able to be reduced more sufficiently at pH value larger than 4.0, and
the electrochemical activity of polyaniline in the phosphate buffer is much higher than
that in 1 mol dm-3 NaCl solution of the pH region of 4.75 and 7.38. Most of
enzymes have an isoelectric point higher than pH 4. When the pH value of the solution is
higher than the isoelectric point, the enzyme carries a negative charge. The above
experimental results indicate that polyaniline still maintains a good electrochemical
activity at pH values higher than 4.0, so the enzymes with negative charges were able to
be doped into polyaniline film during the oxidation process in the phosphate buffer[8,18].
The advantage for the immobilization of an enzyme in this manner is harmless to the
activity of the enzyme, because the phosphate buffer and polyaniline are inert to enzymes,
and polyaniline provides a good charge transfer medium during the enzyme-catalyzed
reaction.
 |
 |
|
Fig.1 The cyclic voltammograms of polyaniline in 1 mol dm-3 NaCl solution, curves: (1) pH 4.57, (2) pH 6.40, (3) pH 7.38, at 20 oC. |
Fig.2 The cyclic voltammograms of polyaniline in 0.2 mol dm-3 phosphate buffer, Curves: (1) pH 4.57, (2) pH 6.40, (3) pH 7.38, at 20 oC. |
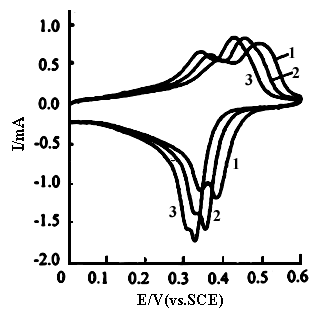
Curves 1 and 2 shown in Fig.3
are the cyclic voltammograms of polyaniline in the solution consisting of 2.5 mol dm-3
ZnCl2 and 3.0 mol dm-3 NH4Cl with pH 4.40 and 4.92,
respectively. The sweep potential range is between -0.10 and 0.80 V. Their are two
oxidation peaks and two reduction peaks on curve 1 at pH 4.40 and two oxidation peaks and
one reduction peak on curve 2 at pH 4.92. Also their peak potentials shift toward negative
potentials with increasing pH value. Compared with Fig.2, the redox currents decrease
quickly with increasing potential when the sweep potential is over 0.5 V(in Fig.3), but
the currents of the oxidation and reduction peaks in Fig.3 are larger than those in Fig.2.
This means that the electrochemical activity of polyaniline in the solution consisting of
ZnCl2 and NH4Cl is larger than that in the phosphate buffer.
For further identification of peak potential shift with pH value, the cyclic
voltammetry was carried out in the solution consisting of 2.5 mol dm-3 ZnCl2
and 3.0 mol dm-3 NH4Cl with three pH values, and the scan potential
was changed to the region of 0 and 0.60 V with changing X axis scale from 0.25 to
0.10 V cm-1. Curves 1,2 and 3 in Fig.4 show the cyclic voltammograms of
polyaniline at pH 4.40,4.75 and 4.92, respectively. Their are two oxidation peaks and two
reduction peaks with a small space on each curve, which are similar to those of
polyaniline in HCl solution with pH < 4 [9], but different from those in
Fig.2. The difference between Fig.4 and Fig.2 is caused by the solution property. The
potentials of two oxidation peaks shift from 0.49 and 0.37 V (curve 1) at pH 4.40 to 0.42
and 0.34 V(curve 3) at pH 4.92, respectively; also the potentials of two reduction peaks
shift from 0.38 and 0.32 V( curve 1) at pH 4.40 to 0.33 and 0.30 V(curve 3) at pH 4.92,
respectively. The space between two reduction peaks decreases more quickly than that of
two oxidation peaks with increasing pH value. This indicates that the reduction of
polyaniline is more sensitive to the proton concentration than the oxidation of
polyaniline. Compared with Fig.3, there is only a reduction peak on curve 2 at pH 4.92.
This is due to a small space between two reduction peaks at pH 4.92, so the X-Y recorder
at the X axis scale of 0.25 Vcm-1 can not distinguish them. However,
when X axis scale was changed from 0.25 V cm-1 to 0.10 V cm-1
for the cyclic voltammograms of polyaniline in 0.2 mol dm-3 phosphate buffer
with different pH values, the results are the same as those in Fig.2. The difference
between Fig.2 and Fig.4 is caused by the property and pH value of the solution. Two pairs
of redox peaks in the cyclic voltammograms are typical of emeraldine salt form of
polyaniline; at higher pH values, the individual oxidation and reduction peaks are merged
into a pair of reduction peaks, this is indicative of emeraldine base form of polyaniline[9].
In the latter, little deprotonation and protonation occur during the redox processes of
polyaniline.
 |
 |
|
Fig.3 The cyclic voltammograms of polyaniline in the solution consisting of 2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl, sweep potential region between -0.10 and 0.80 V(vs.SCE),curves: (1) pH 4.40, (2) pH 4.92, at 20 oC. |
Fig.4 The cyclic voltammograms of polyaniline in the solution consisting of 2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl, sweep potential region between 0.0 and 0.60 V, curves: (1) pH 4.40, (2) pH 4.75, (3) pH 4.92, at 20 oC. |
3.2 Charge-discharge process
The battery for the charge-discharge process consisted of a polyaniline
electrode, a zinc electrode and the solution consisting of ZnCl2 and NH4Cl.
Polyaniline film was polymerized on a platinum foil (1 cm2), the charge passed
was 2.4 C during the electrolysis of aniline. The battery was charged and discharged
between 0.75 and 1.50 V at a constant current of 2 mA. Curves 1 and 2 in Fig.5 show the
charge and discharge processes of polyaniline in the solution consisting of 0.50 mol dm-3
ZnCl2 and 0.90 mol dm-3 NH4Cl with pH 4.40. Both of the
charge time and discharge time are 2.1 min. Curves 3 and 4 show the charge and discharge
processes of polyaniline in the solution consisting of 2.5 mol dm-3 ZnCl2
and 3.0 mol dm-3 NH4Cl with pH 4.40. Both of the charge time and
discharge time are 2.7 min. Curves 5 and 6 show the charge and discharge processes of
polyaniline in the solution consisting of 2.5 mol dm-3 ZnCl2 and 3.0
mol dm-3 NH4Cl with pH 4.92, which was adjusted using aqueous
ammonia. Their charge time and discharge time are 2.9 min. It is clear that both the
charge time and discharge time of polyaniline in the solution consisting of 0.50 mol dm-3
ZnCl2 and 0.90 mol dm-3 NH4Cl with pH 4.40 are the
shortest among the three kinds of the solutions, because of the lowest buffer capacity.
However, a surprising result was observed in the solution consisting of 2.5 mol dm-3
ZnCl2 and 3.0 mol dm-3 NH4Cl, that is, the charge time
and discharge time at pH 4.92 are longer than those at pH 4.40. According to those
results, the charge and discharge capacities of polyaniline at pH 4.92 are increased by
7.4% compared to pH 4.40. To further prove this result, in another experiment using a
thinner film of polyaniline polymerized on the same piece of platinum foil, the charge
consumed was 1.3 C during the electrolysis of aniline. Polyaniline was charged and
discharged between 0.75 and 1.50 V at a constant current of 1 mA. In the solution
consisting of 2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl
, both the charge time and discharge time of polyaniline were 3.25 min at pH 4.40, and
3.50 min at pH 4.92 (charge and discharge curves omitted here), so the charge and
discharge capacities of polyaniline at pH 4.92 are increased by 7.7% compared to pH 4.40.
These results are analogous to those shown in Fig.4, in which the area of the cyclic
voltammogram at pH 4.92 is also larger than that at pH 4.40. This is due to fact that the
buffer capacity of the solution consisting 2.5 mol dm-3 ZnCl2 and
3.0 mol dm-3 at pH 4.92 is higher than that at pH 4.40. It is clear that the
buffer capacity of the solution is very important to the electrochemical activity of
polyaniline, it plays a significant role in not only increasing the charge-discharge
capacity of polyaniline and but also decreasing the corrosion of the zinc electrode in the
polyaniline batteries.
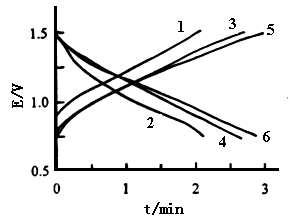 |
Fig.5 The charge-discharge process of polyaniline , curves: (1) and (2) in the solution of 0.5 mol dm-3 ZnCl2 and 0.9 mol dm-3 NH4Cl with pH 4.40, (3) and (4) in the solution of 2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl with pH 4.40, (5) and (6) in the solution of 2.5 mol dm-3 ZnCl2 and 3.0 mol dm-3 NH4Cl with pH 4.92, at 20 oC. |
3.3 Effect of the solution on
electrochromism
During the potential scan, the change in the color of polyaniline film was
observed in the phosphate buffer and the solution containing ZnCl2 and NH4Cl
,at pH > 4. When the potential swept from 0.80 to -0.10 V, the color of polyaniline
film changed from reddish purple to blue, green, and transparent yellow. This phenomenon
is similar to that in 1 mol dm-3 HCl solution[9,16]. To confirm the
change in the color of polyaniline film with the potential , in situ
spectroelectrochemical techniques were used in this study. The cell for this study
consisted of a polyaniline polymerized on an ITO electrode, a platinum gauze , a reference
electrode of Ag/AgCl with saturated KCl solution, and a phosphate solution , which were
put in a quartz container. The potential of polyaniline was changed from 0.60 to -0.20 V.
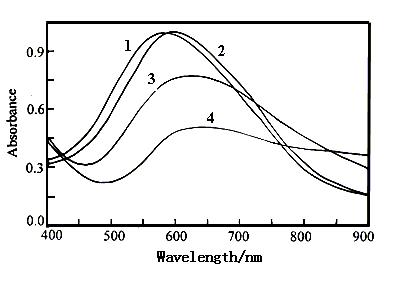
Figure 6 shows the visible spectra of polyaniline film in 1 mol dm-3
NaCl solution with pH 4.57 at various potentials. The absorption peaks occur at 553
nm(curve 1) at 0.60 V and 524 nm(curve 2) at 0.30 V. When the applied potential was
decreased to -0.10 V, the absorbance value of the peak increases only a little , but the
wavelength of the peak still occurs at 524 nm(curve 3). As the applied potential was
decreased continuously to -0.20 and -0.30 V, the absorption peak shifts to 571 nm(curve
4), and 580 nm(omitted here), respectively. From Fig.6, we can see that the wavelength of
the absorption peak changes a little in the potential region of 0.60 and -0.30 V. The film
color was the blue at 0.60 V and the shallow green at -0.30 V. The small potential
dependence of the film color is caused by the very low electrochemical activity(in Fig.1)
of polyaniline, in which no oxidation and reduction peaks occur on the cyclic
voltammograms.
Figure 7 shows the visible spectra of polyaniline film in 0.2 mol dm-3
phosphate buffer with pH 7.38 at various potentials. The absorption peaks occur at 587
nm(curve 1) at 0.60 V, 598 nm(curve 2) at 0.10 V, 625 nm (curve 3) at 0.05 V and 649
nm(curve 4) at 0.0 V. When the applied potential was decreased further, the wavelength of
the absorption peak is only very slightly affected, but the absorbance value decreases
more quickly with decreasing potential. The latter is different from that shown in Fig.6,
so the change of the film color was observed more clearly in this solution than that in 1
mol dm-3 NaCl with pH 4.57.
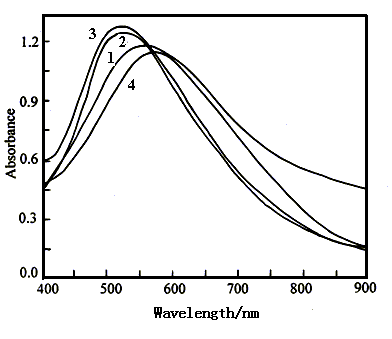 |
Fig.6 The visible spectra of polyaniline film in 1 mol dm-3 NaCl solution with pH 4.57 at various potentials, Curves: (1) 0.60 V, (2) 0.30 V, (3) -0.10 V, (4) -0.20 V. |
|
Fig.7 The visible spectra of polyaniline film in 0.2 mol dm-3 phosphate buffer with pH 7.38 at various potentials, curves: (1) 0.60 V, (2) 0.10 V, (3) 0.05 V, (4) 0.0 V. |
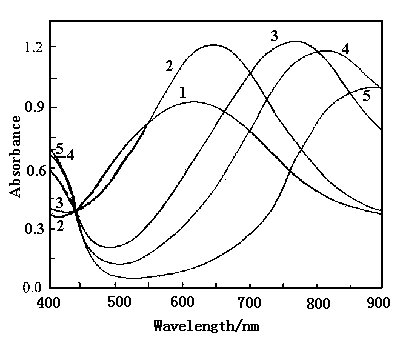
Figure 8 shows the spectra of polyaniline film in 0.2 mol dm-3 phosphate buffer with pH 6.40 at various potentials. The absorption peaks occur at 523 nm(curve 1) at 0.60 V, 556 nm(curve 2) at 0.30 V, 640 nm(curve 3) at 0.20 V, 779 nm (curve 4) at 0.15 V, 818 nm(curve 5) at 0.10 V and 840 nm (curve 6) at -0.20 V. The wavelength of the absorption peak at 0.40 V(omitted) is the same as that at 0.60 V, so the wavelength shift of the absorption peak begins at 0.30 V. Curves 3,4,5 and 6 cross at a common point at 454 nm, in which they have the isoabsorption value . From Fig.8, we can see that the wavelength of the absorption peak shifts from 523 to 840 nm as the potential decreased from 0.60 to -0.20 V. This bathochromic shift is similar to that of polyaniline film polymerized on an ITO conducting glass in 0.1 mol dm-3 HClO4 solution[19]. The color of polyaniline film in 0.2 mol dm-3 phosphate buffer with pH 6.40 changed from reddish purple to blue, green and yellow as decreasing potential from 0.60 to -0.20 V.
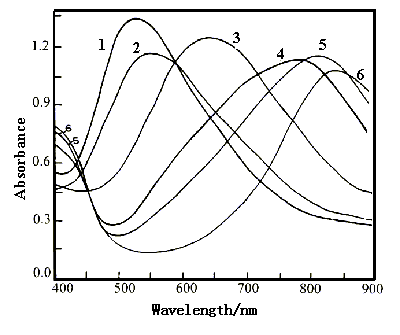 |
Fig.8 The visible spectra of polyaniline film in 0.2 mol dm-3 phosphate buffer with pH 6.40 at various potentials, curves: (1) 0.60 V , (2) 0.30 V, (3) ) 0.20 V, (4) 0.15 V, (5) 0.10 V, (6) -0.20 V. |
|
Fig.9 The visible spectra of polyaniline film in 0.2 mol dm-3 phosphate buffer with pH 4.57 at various potentials, curves: (1) 0.60 V, (2) 0.40 V, (3) 0.30 V, (4) 0.20 V, (5) 0.0 V. |
From Fig.s 7,8 and 9, we can see that the starting potential for the wavelength shift, i.e, the change of the color of polyaniline is 0.10 V at pH 7.38, 0.30 V at pH 6.40 and 0.40 V at pH 4.57.The tendency of this potential shift is similar to those potentials of the redox peaks shown in Fig.2, in which the potentials of the oxidation peak and the reduction peak shift toward the positive potentials with decreasing pH value. Above experimental results indicate that the electrochromism of polyaniline is related to whether the oxidation and reduction peaks occur on the cyclic voltammograms or not, and the starting potential for the change of the color is dependent on the potential values of the oxidation and reduction peaks, which are a function of pH value.
4. CONCLUSION
The electrochemical activity of polyaniline in 1 mol dm-3 NaCl solution
with pH 4.57 is very low and the color of the film changed hardly with the potential while
there are no redox peaks on the cyclic voltammograms. However, polyaniline in 0.2 mol dm-3
phosphate buffer at pH 4.57 and 6.40 has a good electrochemical activity and
electrochromic characteristics , even at pH 7.38, polyaniline still has a certain activity
and electrochromism. This is because there are redox peaks on the cyclic voltammograms.
Thus, whether there are the redox peaks on the cyclic voltammograms or not is a probe for
the change in the color of polyaniline film with potential, and vice versa. No isoesbestic
point exists on the visible spectra of polyaniline film in 1 mol dm-3 NaCl
solution with pH 4.57 in the potential region of 0.60 and -0.20 V. However, there is an
isosbestic point in the visible spectra of polyaniline film in 0.2 mol dm-3
phosphate buffers with pH 6.40 or 4.57. The former occurs at 454 nm in the potential
region of 0.20 and -0.20 V; the latter occurs at 443 nm in the potential region of 0.60
and 0.0 V. It is clear that the potential region for the change of the color depends on
the potential, as well as pH value.
In the solution consisting of 2.5 mol dm-3 ZnCl2
and 3.0 mol dm-3 NH4Cl, the electrochemical activity of polyaniline
increases with increasing pH value from 4.40 to 4.92. This is caused by the buffer
capacity, since the buffer capacity of the solution at pH 4.92 is larger than that at pH
4.40. This plays a very important role in increasing the charge-discharge capacity of
polyaniline and in decreasing the corrosion of the zinc electrode in the polyaniline
batteries.
REFERENCES
[1] Desilvestro J, Scheifele W, Haas O. J. Electrochem. Soc., 1992, 139: 2727.
[2] Wang B C, Li G, Li C Z, Wang F S. J. Power Sources, 1988, 24: 115.
[3] Mu S L, Ye J H, Wang Y H. J. Power Sources, 1993, 45: 153.
[4] Kobayashi T, Yoneyama N, Tamura H. J. Electroanal. Chem., 1984, 177: 281.
[5] Akhtar M, Weakliem H A, Paiste R M et al. Synth. Met., 1988, 26: 203.
[6] Karg S, Scott J C, Salem J R et al. Synth. Met., 1996, 80: 111.
[7] Bartlett P N, Whitaker R G. Biosensor, 1987, 3: 359.
[8] Mu S L, Xue H G, Qian B D. J. Electroanal. Chem., 1991, 304: 7.
[9] Huang W S, Humphrey B D, MacDiarmid A G. J. Chem. Soc., Faraday Trans I, 1986, 82:
2385.
[10] Osaka T, Nakajima T, Naoi K et al. J. Electrochem. Soc., 1990, 137: 2139.
[11] Stilwell D E, Park S M. J. Electrochem. Soc., 1988, 135: 2497.
[12] Tsakova V, Milchev A. Electrochimica Acta, 1991, 36: 1579.
[13] Genies E M, Boyle A, Lapkoski M et al. Synth. Met., 1990, 36: 139.
[14] MacDiarmid A G, Yang L S, Huang W S et al. Synth. Met., 1987, 18: 393.
[15] Mermilliod N, Tanguy J, Hoclet M et al. Synth. Met., 1987, 18: 359.
[16] Genies E M, Tsintavis C. J. Electroanal. Chem., 1986, 200: 127.
[17] Batich C D, Laitinen H A, Zhou H C. J. Electrochem. Soc., 1990, 137: 883.
[18] Wang H Y, Mu S L. J. Electroanal. Chem., 1997, 436: 43.
[19] Kabumoto A, Shinozaki K, Watanable K et al. Synth. Met., 1988, 26: 349.