http://www.chemistrymag.org/cji/2000/023013pe.htm |
|
Liu Yueying, Fu Jinkun#, Zhou Zhaohui#,
Lin Zhongyyu#, Li Renzhong
(Department of Biology, #Department of Chemistry, Xiamen University, Xiamen,
361005,China)
Abstract Highly dispersive supported palladium
catalyst was prepared by the reduction of Bacillus licheniformis R08 biomass. TEM analysis
indicated that R08 biomass was able to adsorb and reduce Pd2+ to Pd0
particle. IR spectrum study showed that -COO- and -HPO42- groups on the cell wall may involve in the process of adsorbing Pd2+.
XPS detection showed that Pd2+ ions on g-Al2O3 were reduced to Pd0 by R08
biomass. The resulted Pd0/g-Al2O3 catalyst after heating treatment
possesses much high efficiency for catalyzing the oxidation of carbon monoxide, comparing
with the catalyst prepared by the common impregnated method. Moreover, TEM
micrograph indicated that the Pd0 particles on the supporter g-Al2O3 were
highly dispersive with mean size of 5nm after heat treatment.
Keywords Palladium catalyst, biosorption, bioreduction, Bacillus licheniformis.
1. INTRODUCTION
The catalytic properties of supported metal catalysts are closely related to the
dispersive degree of metal [1,2]. Therefore, the preparations of highly
dispersed supported metal catalysts have attracted a great deal of attention [3].
In our previous paper, we reported D01 biomass to reduce Au3+ for the
preparation of highly dispersed supported gold catalyst Au/a
-Fe2O3 [4]. As a part
of our research work on the preparations of highly dispersive precious metal catalysts by
microbial method, here we further report the preparation of palladium catalyst, a much
more common used catalyst for hydrogenation and oxidation. In the preparation, the
biosorption of Pd2+ by R08 biomass (a dead biomass) was characterized with TEM
and IR techniques. The Pd/ g-Al2O3
catalyst was prepared from the bioreduction of Pd2+ impregnated on g-Al2O3 and heat
treatment, then characterized by TEM and XPS techniques. The catalyst showed much high
efficiency for the catalytic oxidation of carbon monoxide compared with the common
impregnated catalyst.
2. EXPERIMENTAL
2.1 Bacterial cultivation and biomass preparation
The bacterial strain R08 used in this study was isolated from pit water and identified
as Bacillus licheniformis R08. The strain R08 was cultivated as previously reported [4].
The cultures were harvested by centrifuging (3500 rpm, 15 min) and the cell pellets were
subsequently washed three times with deionized water. The resulted biomass was dried at 60oC
and ground with a mortar and pestle. The dry, dead biomass was stored in a dryer for use.
2.2 Measurement of IR spectra
The blank R08 biomass and the biomass were contacted with PdCl2 solution
for 1 h and then dried at 60oC respectively. Their IR spectra were measured on
740sx FT-IR spectrometer.
2.3 Preparation of Pd0/g-Al2O3 catalyst by impregnated method
Stoichiometric amounts of PdCl2 aqueous solution was impregnated on g-Al2O3 (30-60
meshes, BET surface area 240m2/g) for the preparation of catalyst precursor Pd2+/g-Al2O3. The
loading weight of Pd2+ was 2 %. Pd2+/g-Al2O3 was pre-dried under vacuum at 80oC
for 4 h and heated to 600oC at a rate of 5oC/min in a reaction tube.
The resulted catalyst was kept at 600oC for 1.5 h and reduced with hydrogen at
250oC for 1h for the preparation of Pd0/g-Al2O3 catalyst.
2.4 Preparation of supported Pd0/g-Al2O3 catalyst with microbial
reduction
The above precursor Pd2+ /g-Al2O3, R08 biomass and deionized water were
mixed with weight ratio of 1:0.3:5. The mixture was incubated at 30oC and then
was kept at pH 3.5 for the preparation of Pd0/g-Al2O3. The sample was periodically removed
and detected with a XPS (ESCALRB MKI X-ray photoelectron spectrometer) for the
determination of the reduced degree of the sample until Pd2+ ions supported on g-Al2O3 were
completely reduced to Pd0. The resulted Pd0/g-Al2O3 was heated
in a oven at 600oC for 1.5 h to destroy the biomass adsorbed on the Pd0/g-Al2O3, and
further used for the preparation of TEM micrograph and the test of the catalytic reaction.
2.5 Test of the catalytic activity
The above 2 % Pd0/g-Al2O3 catalysts (0.5 g) were filled in a
fixed bed microreactor (0.6cm i.d.) and heated at 150oC. CO (2%)-air mixture
was flowed with GHSV 20000 ml· g-1·h-1 (bioreduced method) or GHSV 1000 ml · g-1·h-1
(conventional method) for the test of catalytic activity. The activities of Pd0/g-Al2O3 catalyst
prepared by two different methods were compared. The conversion of 2% CO balanced
with air was analyzed according to CO percent content in the concentration of initial and
finial gas by an online ST03 model gas chromatograph with a thermal conductivity detector
and 5Å molecular sieve column.
2.6 Preparation of TEM micrographs
The sample was taken out by the copper grid. The sample on the copper grid was dried
at room temperature and then recorded with a TEM (JEM 100 CXII transmission electron
microscope) at an accelerating voltage of 100 kV.
3. RESULTS AND DISCUSSION
3.1 TEM characterization of adsorption of Pd2+ by R08 biomass
Fig.1-a and 1-b were the transmission electron micrographs of R08 biomass contacted
without and with PdCl2 solution respectively. It can be found that Pd2+ ions
were adsorbed and reduced to metallic palladium by R08 biomass. The obvious reduction of
Pd2+ ions by R08 biomass indicated that R08 biomass could be used as Pd2+
reductant for the preparation of supported palladium catalyst.

Fig.1 Transmission electron micrographs of B. licheniformis R08 biomass
(a) R08 biomass (b) R08 biomass contacted with PdCl2 solution
3.2 IR spectra characterization of R08 biomass
contacted with Pd2+
The IR spectra of the biomass and the biomass contacted with Pd2+solution for 1
h were measured. The bands at 1547 cm-1 and
1402 cm-1 are corresponded to asymmetric
and symmetric vibration of C=O bond respectively. Moreover, two bands at 1230 cm-1 and at 1286 cm-1
may associate with the vibration of n (C-O ) and n (P-O) [5]. The bands of R08 biomass contacted with Pd2+
shifts obviously to lower frequency from 1547 cm-1
to 1539 cm-1, which suggest the existence
of interaction between COO-group of the
biomsss and Pd2+ ions. In addition, the spectral band of n (P-O)
shifts from 1286 cm-1 to 1281cm-1, which may result from the interaction between palladium
and HPO42-group of the biomass [5].
The results are in agreement with some reports on biosorption of metal ions [6,7].
3.3 XPS spectral characterization of reduction of Pd2+/g-Al2 O3
by R08 biomass
The reduced degree of Pd2+ supported on g-Al2 O3 may be monitored according to the
ratio of the total area of spectral peaks for the bonding energy of Pd2+ to Pd0.
The bioreduction of Pd2+/g-Al2O3 by R08 biomass was examined by XPS.
Fig.2-a was the XPS spectrum of catalytic precursor Pd2+/g-Al2O3 contacted
with R08 biomass for 12 h. It may be defined that about 20% of Pd2+ ions were
reduced to Pd0. Fig.2-b shows the XPS spectrum of the sample of Pd2+/g-Al2O3 reduced by
R08 biomass for 48 h. The results indicated that the Pd2+supported on g-Al2O3 were
completely reduced to Pd0.
The above results further supported that R08 biomass could be used as a
reductant for preparation of Pd0/g-Al2O3 and the mechanism of Pd2+ biosorption
involved a reduction of Pd2+ ions. In the bioreduction, the enzymatically
mediated bioreduction of metals has been well documented [8]. However,
non-enzymatically mediated bioreduction of metals including palladium has only
received little or no attention. Brierley et al [9] have indicated that the
biosorption of platinum group metal ions by MAR (a metal removal agent prepared by spent
dead biomass from fermentation processes) followed by an apparent reduction of the metal
ions. Recently, Lloryed et al [8] reported that the resting cells of
Desulfovibrio desulfurcans could reduce enzymatically soluble Pd2+ ions to Pd0,
using pyruvate, formate, or H2 as the electron donor without biochemical
cofactors. The process of Pd2+ reduction was O2 insensitive. Our
results showed that the biosorption and bioreduction of Pd2+ by R08 dead
biomass was a non-enzymatic reaction, in which metabolic process is not involved. The
reduction of Pd2+ by R08 dead biomass may relate with -HPO42-
and -COO- groups on the biomass surface.
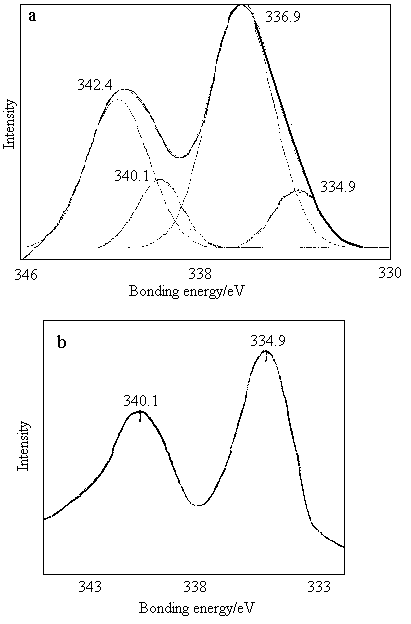 |
Fig.2 XPS spectra of Pd2+/g-Al2O3 contacted
with R08 biomass for different period, (a)12 h, (b) 48h |
3.4 Catalytic activity for CO oxidation and TEM
characterization of Pd0/g-Al2O3 catalyst
Pd0/g-Al2O3
prepared by bioreduced method was used as catalyst for oxidation of CO. The catalyst
showed better performance in the conversion of CO to CO2. The catalytic
activity was 100% with higher GHSV (20000ml·g-1·h-1) at 150oC. The catalytic activity was retained
for more than 70 h. Although Pd0/g-Al2O3 catalyst prepared by conventional
impregnated method also showed 100% conversion efficiency of CO oxidation, the rate of
reaction gas flowed was much low GHSV (1000ml·g-1·h-1), and the activity was gradually decreased to 50% after 34
h.
The supported Pd0/g-Al2O3 catalyst prepared by bioreduced method
were observed by TEM, which showed that Pd0 particles on the supporter g-Al2O3 were
highly dispersive with an average size of 5nm after heat treatment (Fig.3). It seems that
the particles do not enlarge obviously, since there were the separation of biomass between
Pd0 particles. From figure 3, there were many particles adsorbed on supporter g-Al2O3. This
showed that most of the metallic palladium particles were retained and not oxidized.
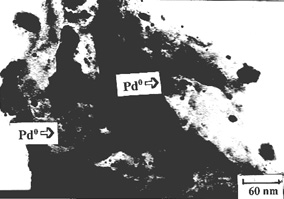 |
Fig.3 The TEM micrograph of Pd0/g-Al2O3 catalyst prepared by bioreduction method |
According to the above results, it is
considered that catalyst precursor Pd2+/g-Al2O3 was completely reduced to Pd0/
g -Al2O3 by
R08 biomass. The biomass on the Pd0/g-Al2O3 has been destroyed and Pd0
on g-Al2O3supporter
was fully exposed after the heat treatment. Moreover, due to the in-situ reduction
of Pd2+ ions by R08 biomass supported on g-Al2O3 under the normal temperature and the
"anchor action" of
biomass adsorbed on the Pd0/g-Al2O3[10], those effects will
reduce the shift and gathering of Pd0 particles on g-Al2O3 supporter during the heating
treatment. Because the Pd particles on the supporter were nano-particles and highly
dispersive, the catalyst Pd0/g-Al2O3 prepared with bioreduced method shows
relatively high catalytic activity and long activity life for oxidation reaction of carbon
monoxide. The reaction temperature (150oC) of catalytic reaction was much lower
than that of the catalyst Pd0/g-Al2O3 (the load of Pd2+ for 5%)
prepared with conventional method reported in literature (230oC)[11].
Under the same Pd2+ load weight, the supported catalyst Pd0/g-Al2O3 prepared
with R08 biomass reduction is of much high catalytic activity and long activity life than
the catalyst prepared by the conventional method. The differences in the catalytic
activity may be resulted from the dispersity and size of Pd0 particles on the
supporter g-Al2O3.
Therefore this method is of potential application on the preparation of highly dispersed
precious metal catalyst.
REFERENCES
[1] Hao Z P, An L D, Wang H L. J. Mol. Catal. (Fenzi Cuihua), 1996, 10 (3): 235-240.
[2] Minicò S, Scirè S, Visco A M et al. Catal. Lett., 1999, 47: 273-276.
[3] Henry C R. Surface Sci. Reports, 1998, 31 (7-8): 235-325.
[4] Liu Y Y, Fu J K, Hu R Z et al. Acta Microbiol. Sinica (Weishengwu Xuebao),
1999, 39 (3): 260-263.
[5] Wu J G. Modern Fourier-transform infrared spectroscopy and its application (Part II).
Beijing: Literature of Science and Technology Press, 1994.
[6] Kuyucak N, Volesky B. Biotechnol. Let., 1988, 10 (2): 137-142.
[7] Chen Y S, Sun Q J, Chen J et al. Adv. Enveron. Sci. (Huanjin Kexue Jingzhan), 1997, 5
(6): 34-43.
[8] Lloyd J R, Yong P, Macaskie L E. Appl. Environ. Microbiol., 1998, 64 (11): 4607-4609.
[9] Brierley J A, Vance D B. Proc. Int. Symp., 1987 (Pub.1988), 477-485.
[10] Fourest E, Roux J C. Appl Microbiol Biotechnol., 1992, 17: 399-403.
[11] Luo M F, Hou Z Y, Yuan X X, Zheng X M. Catal. Lett., 1998, 50: 205-209.