http://www.chemistrymag.org/cji/2000/029043pe.htm |
Sep.30, 2000 Vol.2 No.9 P.43 Copyright |
Magnetic field effects on the polymerization of natural plant powder with silicic acid*
Ma Wei, Xiao Jin#, Guo Liyan ,Yang Minxia, Chen Jing(Dalian University of Technology, Dalian 116024; #South China University of Technology, Guangzhou 510641 )
Received Jan.15, 2000; Supported by National Science Foundation of China (29777011).
Abstract Effects of magnetic field on the
plant powder polymerization with silicic acid have been studied. The result of IR and
viscosity coefficient test shows that the reaction rates, molecular weight and structure
of products are changed with magnetic field.
Keywords natural polymer modification, magnetic field, silicic
Resource of the affergrowth has attracted attention in the world, and the study of watertreatment agent of natural polymer modification becomes hot spot [1]. The activity of silicic acid has functions of flocculation and restrain on biology toxicity of aluminum in sourish solution. Some research workers studied the effects of magnetic field on polymeric reaction [2,3], but the effects on the natural plant powder in polymerization with silicic acid have not been reported yet. However, the water treatment agent synthesized of the two raw materials has good purifying property [4]. So the effects of magnetic field on the plant powder in polymerization with silicic acid are studied in this paper.
1 EXPERIMENTAL
1.1 Materials
F691 is a type of natural polymer (plant gum) from south China. The components and the
molecular weight were tested by chemical separation and gelatin chromatogram (GPC) and the
result is shown in Table 1.
Component |
Cellulose |
Lignin |
Tannin |
Polysaccharide |
Ecty |
other |
Totals |
Molecular weight (n) |
/ |
78000 |
2600 |
910000 |
38000 |
/ |
/ |
Content % |
48.12 |
19.08 |
6.38 |
20.67 |
3.65 |
2.10 |
100 |
1.2 Instruments
The instruments we used are the magnetizing equipment (made of Nd-Fe-B, 0.3T), pH
meter model XL-9401A, magnetic stirrer model 78-1, viscosity meter and IR
spectrophotometer of Analect model RFX-65A.
1.3 Preparation of the water treatment agent
Based on the preparation method of CGA[5] , ethanol was added
to the plant powder as dispersing agent. 30% sodium hydroxide was used for the
basification. Sodium silicate and monochloroacetic acid were used for
aetherization for 30min at 40-70¡ãC. After 8 hours the mixture was diluted to 10% of the
available component and the pH was adjusted to 7.8 by water and hydrochloric acid.
The agent obtained without magnetic field was called CMS1 and that with magnetic field of
0.3T was called CMS2.
2 RESULTS AND DISCUSSION
2.1 General properties
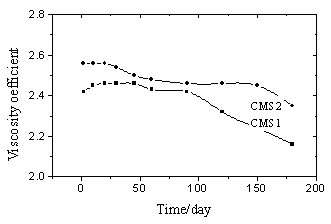
The agent is a brown color liquid, with slight plant smell and good solubility. The average molecular weight is about 9¡Á105-1¡Á106. By means of appraisal of the polymer flocculent, the viscosity coefficient of the agent with 0.02% concentration was tested in different time (compared to distilled water). The result is shown in Fig.1.
 |
 |
| Fig.1 The viscosity coefficient changes with time (25¡ãC) | Fig.2 IR Spectrum of the products |
Figure 1 shows that the viscosity
coefficient of the product with magnetic field (CMS2) is somewhat larger than that of CMS1
(without magnetic field). It indicates that the molecular weight of CMS2 is larger than
that of CMS1. The reaction rate with magnetic field is larger than that without magnetic
field, The viscosity coefficient of CMS1 achieves maximum ten days later. This tendency is
more enhanced in other sample.
2.2 IR spectrum of the agent
The IR absorption spectrum is shown in Fig.2 and table 2-
table 4.
Table 2 IR spectrum of F691
Wavenumber (cm-1) |
Group and
Class ring in Cycloalkanes C-O stretching, dimer, Amide, CH2-O-H CH2 C-H aryl vibration CH2 OH vibration aryl vibration amide, C=O stretching Dimeric, carboxylic, C=O stretching, aryl C-H acylimide C-N CH3, CH2 stretching Broad O-H stretching |
| Wavenumber (cm-1) 590.1 819.6 879.48 1049.1 1085.7 1322.9 1411.6 1625.96 2935.1 3421.1 |
Group and Class ring in Cycloalkanes Si-CH3 rocking Si-C stretching C-CO-C-CO-C stretching Si-O-Si stretching CH2 C-O-H C-O ¨C H stretching Amide, C=O stretching CH3, CH2 stretching Broad O-H stretching |
Table 4 IR spectrum of CMS2
| Wavenumber
(cm-1) 553.47 669.18 771.39 833.10 863.95 931.45 1095.37 1251.58 1282.43 1427.07 1600.63 2360.44 2973.70 3405.67 |
Group and
Class ring in Cycloalkanes amide NH out ¨Cof ¨Cplane wagging Si-CH3 rocking Si-OH strtching, Si-C stretching Si-H bending and wag Si-O-Si asym.stretching C-O-CO asym.. stretching C-O stretching, dimer C-O-H in-plane bending amide SiH, SiH2, stretching, CH3, CH2 stretching Broad O-H stretching |
2.3 Discussion
Table 2 shows that the groups of the polysacchand (contain cellulose), dextrose, xylogen, fragrant, acidamide, ¨COH, etc. exist in the F691 powder. Fig. 2, table2 and table3 show that the groups of carboxylic acid, Si-O-Si and Si-C increase in the product without magnetic field. Fig. 2, table3 and table 4 show that groups of the C-H annulus vibration disappeared, and Si-CH3, Si-O, Si-OH, Si-H (SiH)increased and other group takes transfer in the product with magnetic field. That shows magnetic field changes the structure of the product from polymerization.
3 CONCLUSIONS
Our preliminary results show that the existence of the magnetic field favour the polymerization of natural plant powder with silicic acid. IR and viscosity results indicate the reaction rates, molecular weight and structure of products, also depends on the magnetic field.
Acknowledgements
The authors wish to express their thanks to Mr. Li Jing from the Institute of Chemistry of the Chinese Academy of Sciences, for his assistance in obtaining the GPC and IR data. REFERENCES
[1] Susumu K. Journal AWWA, 1991, (10): 88.
[2] Zhou C H, Su Z X. Chemical journal of Chinese universities, 1981, 2 (3): 372.
[3] Chen Z Y, Zhu X L. Journal of Lanzhou university (Natural Sciences), 1995, 31 (2): 160.
[4] Ma W, Guo L Y, Xiao J et al. Environmental science (Huanjing Kexue), 1999, 1: 59.
[5] Xiao J. Patent of China, ZL.89, 1,03028.X, 1989.¡¡