http://www.chemistrymag.org/cji/2000/02a044pe.htm |
Oct. 1, 2000 Vol.2 No.10 P.44 Copyright |
Study on supramolecular chemistry of calixarenes (III): liquid membrane transport of Fe3+ ions with calixarenes and their derivatives as carriers
Wang Li, Sun Hongbao, Fu Haiying, Jiang
Zhongliang, Shi Xianfa
(Department of chemistry, Tongji University, Shanghai, 200092; State Key Laboratory of
Coordination Chemistry, Nanjing, 210093, China )
Received May 29, 2000; Supported by the National Natural Science Foundation of China (No.29971023)
Abstract The transport of Fe3+
ions with p-tert-butyl calix[n]arenes and their derivatives as carriers was
studied by means of a bubbling pseudo-emulsion liquid membrane. The factors affecting the
transport and the selective transport for Fe3+ ions from a mixture of Fe3+
and Cu2+ ions were investigated. It has been discovered that the gradient
of the acidity between the source phase and the receiving phase is the driving force for
the transport. Additionally, the transports using a series of calixarenes and their
derivatives as carriers were studied and compared with each other. A preliminary
discussion for the transport mechanism was also given in this paper.
Keywords calixarenes, pseudo-emulsion liquid membrane, Fe3+ ions,
transport
Considerable efforts have been directed toward the studies of a new-type receptor calixarenes and their derivatives in the recent years. Known as the third generation of the macromolecules, calixarenes have many special supramolecular characteristics such as molecular recognition, inclusion ability, enzyme catalysis activity etc. Compared with the considerably abundant researches of the alkali or alkaline earth metals in this field, the researches of the transition metals are relatively limited. The element Fe has an important physiologic function in organism. It not only plays an important role in the transport of oxygen and carbon dioxide, but also is an indispensable element in some enzyme and redox systems. So the studies of the interaction of the ferrous ions and calixarenes and their supramolecular chemistry have gained more and more attentions. Up to date, the researches mainly were focused on the coordination interaction between ferrous ions and calixarenes and the structure of their coordinates.[1-4] Liquid membrane transport effect is one of the typical supramolecular chemistry characteristics of the calixarenes. The researches on the membrane transport with calixarenes as carriers have attracted much attention because of their potential application in many fields, but there is only a little work found in the literature.[5, 6] Our research group have reported the transports of K+, Na+ ions with calixarenes as carriers.[7] In this paper, the transport of Fe3+ with calixarenes and their derivatives as carriers was investigated.
1 EXPERIMENTAL SECTION1.1 Instrument and reagents
A specially designed apparatus of a bubbling pseudo-emulsion liquid membrane system, illustrated in Fig.1, was used in this study. Other instruments used include: A pH meter (Shanghai Leici Instrument Factory, Type PHs-3F), UV-VIS spectrophotometer (The Third Shanghai Analysis Instrument Factory, Type 731), de-ionized water fabricator (Shanghai Hezi Medical Instrument Factory, Type 70) and an air pump.
The p-tert-butylcalix[n]arenes (n=4,6,8) 1 and their derivatives calix[n]arene ester 2, calix[n]arene acid 3£¨shown in Fig.2 respectively£©were prepared according to methods already reported.[8-10] All these compounds were characterized by IR, NMR and elemental analysis.[#] Other chemicals used were of reagent grade. Water used in this study were de-ionized (with the conductivity of 1¡Á10-6s - 1¡Á10-7s).
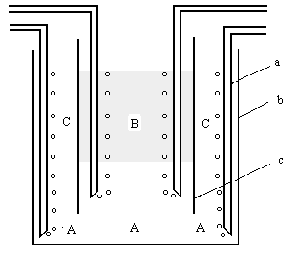 |
 |
|
Fig.1 Liquid
membrane transport cell |
Fig.2
Structure of the calixarenes and their derivatives |
1.2 Experimental methods for
transport
The transports were conducted through the bubbling pseudo-emulsion liquid membrane system
described in detail previously.[7] In this paper, the volumes of the source and
receiving phases were both 75mL and the membrane phase was 100mL mixture of CH2Cl2
and CCl4 (V/V=1:3) in which the carriers were dissolved.
The concentration of Fe3+ ion was determined according to
the concentration of Fe2+ (the Fe3+ ions were reduced to Fe2+
ions) which was measured by means of the UV-VIS spectrometric analysis using o-phenanthroline
as chromogenic agent.
According to the experimental results, the transport time was
determined as 4.5h and the effect of temperature on the transport results can be ignored
within the range of 15-30¡ãC. Therefore the transport experiment was conducted at room
temperature without special control of temperature. For all the transport experiments, the
bubbling was carefully controlled with constant speed.
At each transport experiment, a contrast experiment without carrier in
the membrane phase was performed. All the results reported here are the net transport
results, after deduction of the corresponding blank value from original experimental
result, and they are an average of more than three times transport experiment results.
2 RESULTS AND DISCUSSION
2.1 Transports of Fe3+ using p-tert-butyl calix[6]xarene hexaacetic acid
as carrier
Transport experiments were carried out under a series of different conditions using p-tert-butyl
calix[6]xarene hexaacetic acid as carrier. The conditions changed were the initial pH
gradient between the source and the receiving phase and the initial Fe3+ ion
concentration in the two phases. The results were shown in table 1 and table 2
respectively.
Table 1 The effect of different initial DpH between the two phases on transport
0.78 |
1.44 |
1.96 |
2.26 |
2.44 |
|
net transport: |
0.34 |
1.98 |
4.98 |
11.17 |
17.18 |
Conditions: the source phase: C0Fe3+ = 1.84 ¡Á10-2mol.L-1; C0H+ = 1.10 ¡Á10-2mol.L-1
the receiving phase: C0Fe3+
= 0 mol.L-1; with different initial
pH
(C0Fe3+: the initial
concentration of Fe3+ ions; C0H+ : the initial concentration of H+ ions)
The results in table 1
show the effect of the pH gradient between the source phase and the receiving phase. The
greater the initial pH gradient, the more Fe3+ ions transported into the
receiving phase.
Table 2 presents the results of experiments under different initial
concentration gradient of Fe3+ between the source phase and the receiving
phase. Transports were performed with different initial concentrations of Fe3+
ions in the receiving phase, while that in the source phase is the same. It was noticed
that there was also efficient transport although the difference of Fe3+ concentration
between the two phases is small, even the concentration of Fe3+ in receiving
phase is bigger than that in source phase, if there was a sufficient pH gradient between
the two phases. That is to say, there can be an inverse concentration gradient transport
if the pH gradient between the two phases is big enough.
Table 2 Transport of Fe3£«under different initial DCFe3+ between the two phases
| initial concentration of Fe3+in source phase : C0Fe3+/10-4 mol.L-1 | initial concentration of Fe3+in receiving phase : C0Fe3+/10-4 mol.L-1 | net
transport |
1.84 |
0.00 |
4.30 |
1.84 |
1.09 |
5.15 |
1.84 |
1.67 |
6.87 |
1.84 |
2.32 |
4.30 |
Conditions: the initial pH gradient between the two phases is 2.39.
According to the data in
table 1 and table 2, the conclusion can be drawn that the driving force for transport is
the pH gradient between the receiving and the source phase.
Selective transports of Fe3+ from the mixture of Cu2+
and Fe3+ ions were also studied using p-tert-butyl calix[6]xarene
hexaacetic acid as carrier. The source phase contains different ratio of Cu2+
and Fe3+. The pH gradient between the receiving and the source phase is big
enough.
Table 3 Selective transport of Fe3+ from the mixture of Cu2+ and Fe3+ ions
| initial concentration of H+ C0H+/mol.L-1 |
initial ion concentration in source phase C0/10-3 mol.L-1 |
net transport of Fe3+,Cu2+* DC/10-5 mol.L-1 |
|||
source phase |
receiving phase |
Fe3+ |
Cu2+ |
Fe3+ |
Cu2+ |
| 7.94¡Á10-3 | 2.00 |
9.22 |
7.58 |
3.18 |
0.01 |
| 7.76¡Á10-3 | 2.00 |
9.22 |
0 |
4.98 |
_ |
| 7.76¡Á10-3 | 2.00 |
0 |
7.58 |
_ |
0.01 |
From the data in table 3, it can be seen that the p-tert-butyl calix[6]xarene haxaacetic acid shows an excellent selective transport for Fe3+ ions over Cu2+ ions. This can be explained by the HSAB-theory. The binding atom of p-tert-butyl calix[6]xarene hexaacetic acid is the oxygen atom, which is hard base, and the substrate Fe3+ ion is hard acid while the Cu2+ ion is soft acid. According to the HSAB-theory, the carrier p-tert-butyl calix[6]xarene hexaacetic acid should be an excellent receptor for Fe3+ ion, which can recognize and transport Fe3+ ions with a higher selectivity over Cu2+ ions in the mixture of Fe3+ and Cu2+ ions.
2.2 Transport of Fe3+ using
different calix[n]arenes and their derivatives as carriers
Different calixarenes or their derivatives exhibit different transport abilities for Fe3+
ions under the same conditions. The data, shown in table 4, give us an
order of their transport abilities. The order of different type derivatives of the same
calixarenes is as follows: acid derivatives > calixarenes >ester derivatives. As for
the calixarenes with different degree of polymerization, the order is calix[6]arenes >
calix[n]arenes (n=4,8).
Table 4 Transport of Fe3+ with different calix[n]arenes and their derivatives as carriers
| Carriers | calix[n]arenes n |
ester derivatives n |
acid derivatives n |
4, 6, 8 |
4, 6, 8 | 4, 6, 8 | |
| net
transport DCFe3+/10-5mol.L-1 |
0.601, 1.12, 0.516 | 0.440, 0.773, 0.516 | 3.87, 5.15, 0.687 |
Conditions: the source phase: C0Fe3+ = 9.22
¡Á10-3mol.L-1; C0H+= 7.94 ¡Á10-3mol.L-1
the receiving phase: C0Fe3+
= 0.00 mol.L-1; C0H+ = 2.00 mol.L-1
(C0Fe3+: the initial
concentration of Fe3+ ions; C0H+: the initial concentration of H+ ions)
The transport ability is obviously different when the degree of polymerization is varied. Among the same derivatives, calix[6]arenes exhibit the highest transport abilities, which can be interpreted that the Fe3+ ions match the cavities of calix[6]arenes most and the flexibility of calix[6]arenes make it easier to form the octahedral conformation. On the other hand, among the calixarenes and the derivatives with the same degree of polymerization, the acid derivatives show the best transport abilities. This may be explained by the transport mechanism, which is a proton-coupled co-transport with a flow of protons in the opposite direction. [7] So the calixarenes or their derivatives containing the exchangeable H+ ions have excellent transport abilities. This interpretation is also consistent with the results in table 1 and table 2. The block diagram in Fig.3 can vividly describe the process of the transport. From all the results it can be drawn that the transport mechanism and the transport rule for Fe3+ ions show some analogy to that for K+, Na+ ions described by our previous paper. [7]

Fig.3 Abridged illustration of the transport mechanism
3 CONCLUSION
For all the calixarenes and their derivatives investigated,
the driving force for transport is the pH gradient between the receiving and the source
phase. If the pH gradient is big enough, there can be an inverse concentration gradient
transport between the source and the receiving phase. Among all carriers, the p-tert-butyl
calix[6]xarene hexaacetic acid is the best carrier for Fe3+ ions and it has a
high selective transport for Fe3+ ions over Cu2+ ions. The order of
transport ability is acid derivatives > calixarenes >ester derivatives and
calix[6]arenes > calix[n]arenes (n=4,8).
[#]
Characterized data for the calixarenes and their derivatives:
(1) Compound 1(4) (R = H, n = 4):
IR ( KBr ) n/cm-1 3173
(OH), 1363 (C (CH3) 3)
1HNMR d/ppm 6.77
(8H, ArH), 1.15 (36H, C (CH3) 3)
Anal. Calcd. for C44H56O4: C, 81.48; H, 8.64. Found: C,
81.07; H, 8.54
(2) Compound 1(6) (R = H, n
= 6):
IR (KBr) n/cm-1 3134
(OH), 1363 (C (CH3) 3)
1HNMR d/ppm 6.77
(12H, ArH), 1.15 (54H, C (CH3) 3)
Anal. Calcd. for C66H84O6.CHCl3:
C, 73.66; H, 7.79; Cl, 10.47. Found: C, 73.74; H, 7.95; Cl,9.76.
(3) Compound 1(8) (R = H, n
= 8):
IR (KBr) n/cm-1 3232
(OH), 1363 (C (CH3) 3)
1HNMR d/ppm 6.77
(16H, ArH), 1.15 (72H, C (CH3) 3)
Anal. Calcd. for C88H112O8: C, 81.48; H, 8.64. Found: C,
81.33; H, 8.41
(4) Compound 2(4) (R = CH2COOC2H5,
n = 4):
IR (KBr) n/cm-1 1764
(C=O), 1370 (C (CH3) 3)
1HNMR d/ppm 6.77
(8H, ArH), 4.75 (8H, ArCH2Ar), 4.15 (16H,OCH2CO, CH2Me),
1.23 (12H, CCH3), 1.10 (36H, C (CH3) 3)
Anal. Calcd. for C60H80O12: C, 72.58; H, 8.06. Found: C,
72.30; H, 7.83
(5) Compound 2(6) (R = CH2COOC2H5,
n = 6):
IR (KBr) n/cm-1 1764
(C=O), 1370 (C (CH3) 3)
1HNMR d/ppm 6.95
(12H, ArH), 4.60 (12H, ArCH2Ar), 4.20 (24H,OCH2CO, CH2Me),
1.30 (18H, CCH3), 1.10 (54H, C (CH3) 3)
(6) Compound 2(8) (R = CH2COOC2H5,
n = 8):
IR (KBr) n/cm-1 1757
(C=O), 1376 (C (CH3) 3)
1HNMR d/ppm 6.90
(16H, ArH), 4.50 (16H, ArCH2Ar), 4.15 (32H,OCH2CO, CH2Me),
1.30 (24H, CCH3), 1.00 (72H, C (CH3) 3)
Anal. Calcd. for C120H160O24: C, 72.58; H, 8.06. Found:
C, 73.00; H, 8.18
(7) Compound 3(4) (R = CH2COOH,
n = 4):
IR (KBr) n/cm-1 1750
(C=O), 3395 (OH)
1HNMR d/ppm 6.90
(8H, ArH), 4.80 (8H, ArCH2Ar), 3.20 (8H,OCH2CO), 1.00 (36H, C (CH3)
3)
Anal. Calcd. for C52H64O12·CH2Cl2:
C, 68.99; H, 6.89. Found: C, 68.64; H, 6.99
(8) Compound 3(6) (R = CH2COOH,
n = 6):
IR (KBr) n/cm-1 1748
(C=O), 3392 (OH)
1HNMR d/ppm
7.50-6.90 (12H, ArH), 4.50 (12H, ArCH2Ar), 3.60 (12H,OCH2CO), 1.00
(54H, C (CH3) 3)
Anal. Calcd. for C78H96O18·CH2Cl2:
C, 68.99; H, 6.89. Found: C, 68.24; H, 7.51
(9) Compound 3(8) (R = CH2COOH,
n = 8):
IR (KBr) n/cm-1 1746
(C=O), 3447 (OH)
1HNMR d/ppm 6.90
(16H, ArH), 4.50 (16H, ArCH2Ar), 4.30 (16H,OCH2CO), 1.00 (72H, C (CH3)
3)
Anal. Calcd. for C104H128O24: C,
70.92; H, 7.32. Found: C, 69.42; H, 7.10
[1] Yilmaz M, Vural U S. Syth. React. Inorg. Met-Org. Chem., 1991, 21 (8): 1231.
[2] Jacoby D, Floriani C, Chiesi-Villa A et al. J. Chem. Soc. Dalton Trans., 1993, 813.
[3] Chawla H M, Hooda U, Singh V. Syth. React. Inorg. Met-Org. Chem., 1996, 26 (5): 775.
[4] Beer P D, Keefe A D, Drew M G B. J. Organomet. Chem., 1989, 378 (3): 437.
[5] Izatt S R, Hawkins R T, Christensen J J et al. J. Am. Chem. Soc., 1985, 107: 63 .
[6] Izatt R M, Lamb J D, Hawkins R T et al. J. Am. Chem. Soc., 1983, 105: 1782.
[7] Ye Z F, Wang Y P, Liu Y S et al. J. Membrane. Sci., 1999, 163: 367.
[8] Gutsche C D, Dhawan B, No K H et al. J. Am. Chem. Soc., 1981, 103: 3782.
[9] Arnaud-Neu F, Collins E M, Deasy M et al. J. Am. Chem. Soc., 1989, 111: 8681.
[10]Chang S K, Cho I. J. Am. Chem. Soc. Perkin Trans. 1, 1986, 211.
¡¡