http://www.chemistrymag.org/cji/2000/02a046ne.htm |
Oct. 1, 2000 Vol.2 No.10 P.46 Copyright |
Ji Mian, Gao Shengli, Hu Rongzu, Shi Qizhen
( Department of Chemistry, Northwest University, Xi'an, 710069, China)
Received Apr. 25, 2000; Supported by the National Natural Science Foundation of China (No. 29871023)
Abstract The thermokinetics of the
formation reaction of zinc histidine are studied by a microcalorimeter. On the basis of
experiment and calculated results, three thermodynamic parameters (the activation
enthalpy, the activation entropy and the activation free energy), the rate constant and
three kinetic parameters (the activation energy, the pre-exponential constant and the
reaction order) have been obtained. The influence of temperature and the synthetic
conditions of the complex have been discussed.
Keywords Histidine, Zinc acetate, Microcalorimeter, Thermokinetics
1 INTRODUCTION
Zinc is one of the essential trace elements for human body. L-α-amino acids are the basic structural
units of protein. The complexes of zinc with histidine residues are widely existed in zinc
enzymes and zinc figure proteins. Zinc L-α-histidine complex as additives have widely applied prospects in
medicines, foodstuff and cosmetics [1,2].Our research group have investigated
the solubility properties of Zn(OAc)2-His-H2O systems at 25°C in
the whole concentration range by phase equilibrium method [3]. The result
indicates that there is a region of Zn(His)(OAc)2.1/2H2O
in the diagram and the complex is a congruity soluble one. While the thermokinetics study
of this complex has not been reported in the literature. In this paper, the reaction
thermokinetics of Zn(OAc)2.2H2O with
solution of histidine has been investigated by a microcalorimeter.
2 EXPERIMENTAL
2.1 Materials
Zn(OAc)2.2H2O
is AR grade (Xi'an Chemical Company). L-α-histidine is BR grade (Shanghai Kangda Company) with the >99.5%
purity. The conductivity of the deionized water is 5.48×10-8S.cm-1.
2.2 Experimental equipment and conditions
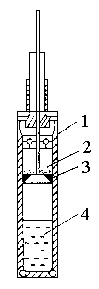
The reaction thermokinetics was studied by a microcalorimeter, type RD496-III (China,
Southwest Institute of Electronic Engineering), which was equipped with two 16 mL vessels
(Fig. 1, where 1 is the calorimetric cell, 2 is the solid sample, 3 is the spacer, 4 is
the solution.). After reaching equilibrium, the spacers of the sample and reference
vessels were pushed down simultaneously and the samples were mixed. The microcalorimeter
was calibrated by Joule effect and its sensitivity were 63.994±0.042
mV.mW-1,
64.308±0.027 mV.mW-1 and 64.499±0.040 mV.mW-1 at the experimental
temperature of 298.15±0.005K, 303.15±0.005K
and 308.15±0.005K, respectively. The experimental precision and
accuracy were checked by measuring the enthalpy of special purity crystalline KCl in
deionized water at 298.15K. The experimental value of Dsol Hqm
of 17.238±0.048 kJ.mol-1 (t
inspection, 99% believability) is excellent agreement with that of Dsol Hqm
of 17.241±0.018 kJ.mol-1
reported in the literature [4].This indicates that the device used in this work
was reliable.
 |
|
| Fig. 1 Device used for the study
1.calorimetric cell; 2. solid sample, 3. Spacer, 4. solution |
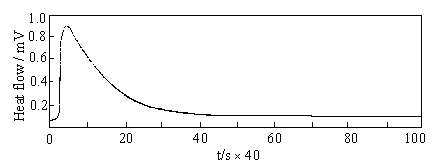
Fig. 2 Typical
thermokinetic curve of the reaction |
3 RESULTS AND DISCUSSION
The reaction could be represented by the following equation (1):
Zn(OAc)2.2H2O (s) + His (aq)
![]() Zn(His)2+ (aq) + 2OAc- (aq) + 2H2O (l)
(1)
Zn(His)2+ (aq) + 2OAc- (aq) + 2H2O (l)
(1)
Within the range of the experimental temperature, the reaction is an exothermic one. The
typical thermokinetic (TK) curve of the reaction was shown in Fig.2. The original data
obtained from the TK curve were shown in Table 1. These experimental data were used in
equation (2) and the reaction order and rate constant were obtained.
ln [(dHt/dt)/H0] = ln k + n ln [1-(Ht/H0) ]
(2)
Where H0 was the total reaction heat (corresponding to the global area under
the TK curve); Ht, the reaction heat in a certain time (corresponding to the
partial area under the curve); dHt/dt, the exothermic rate at time t; k, the
rate constant of reaction; n, the reaction order .
The values of k and n obtained by Eq. (2), the values of E and A
obtained by Eq. (3), the value of DG0![]() obtained by Eq.
(4) and the values of DH0
obtained by Eq.
(4) and the values of DH0![]() and DS0
and DS0![]() obtained by Eq.
(5), which were all listed in Table 2.
obtained by Eq.
(5), which were all listed in Table 2.
ln k = ln A - [E/RT]
(3)
DG0![]() = RT ln [RT/Nhk]
(4)
= RT ln [RT/Nhk]
(4)
ln [k/T] = -(DH0![]() /RT)+ (DS0
/RT)+ (DS0![]() /R)+ ln [kB/h]
(5)
/R)+ ln [kB/h]
(5)
where A was the pre-exponential constant; E, the apparent activation
energy; R, the gas constant; T, the absolute temperature; DG0![]() , the activation
free-energy; N, Avogadro number; h, Planck's constant; DH0
, the activation
free-energy; N, Avogadro number; h, Planck's constant; DH0![]() , the activation
enthalpy; DS0
, the activation
enthalpy; DS0![]() , the activation
entropy; kB, Boltzmann's constant.
, the activation
entropy; kB, Boltzmann's constant.
Table 1 Thermographic data of the reaction
|
298.15K |
298.15K |
303.15K |
303.15K |
308.15K |
308.15K |
50 |
41.35 |
0.109 |
38.63 |
0.117 |
40.40 |
0.123 |
100 |
37.80 |
0.246 |
36.45 |
0.269 |
36.32 |
0.278 |
150 |
32.42 |
0.367 |
31.04 |
0.405 |
30.46 |
0.414 |
200 |
27.38 |
0.472 |
25.25 |
0.521 |
24.75 |
0.529 |
250 |
22.91 |
0.561 |
20.57 |
0.618 |
19.62 |
0.624 |
300 |
19.05 |
0.637 |
16.25 |
0.698 |
15.21 |
0.702 |
350 |
15.69 |
0.702 |
12.58 |
0.764 |
11.51 |
0.766 |
400 |
12.78 |
0.756 |
9.39 |
0.819 |
8.37 |
0.818 |
450 |
10.28 |
0.802 |
6.67 |
0.863 |
5.77 |
0.860 |
500 |
8.13 |
0.840 |
4.29 |
0.898 |
3.57 |
0.894 |
H0 = 1.55, 1.28 and 1.29 J.
Table 2 Thermographic data of the reaction
|
Eq.(2) |
Eq.(3) |
Eq.(4) |
Eq.(5) |
||||||
| k/s-1 | n | r# | E /kJ.mol-1 |
logA /s-1 | r# | DG0 /kJ.mol-1 |
DH0 /kJ.mol-1 |
DS0 /J.mol-1K-1 |
r# | |
298.15 |
31.81 |
0.96 |
0.999 |
22.49 |
5.45 |
0.956 |
64.45 |
19.96 |
-149.0 |
0.945 |
303.15 |
39.92 |
1.02 |
0.993 |
65.00 |
||||||
308.15 |
42.69 |
1.14 |
0.992 |
65.94 |
||||||
# r, the correlation coefficient.
The results in Table 2
clearly indicate that the higher the temperature of the reaction, the faster the rate of
the reaction and the titled reaction is of the first order. While the values of E and DH0![]() were very low and DS0
were very low and DS0![]() is high. These facts showed that the titled reaction easily took
place in the temperature range of 298.15K-308.15K.
is high. These facts showed that the titled reaction easily took
place in the temperature range of 298.15K-308.15K.
The final solution collected together from each experiment and the
solution with the same mole rate as the reaction were concentrated on a 70-80°Cwater bath
till crystal membrane formed on the surface, and then, this was put into a containing P4O10
desiccator to remove trace water. The analytic results indicated that they had the same
composition of Zn(His)(OAc)2.1/2H2O
which is consistent with the result of the literature [3]. The titled complex
can be prepared easily at mild conditions and that is why the complex of Zn(His)(OAc)2.1/2H2O
has a high enthalpy of formation (-1665.02±3.28 kJ. mol-1)
[5].
REFERENCES
[1] Taguchi M, Inokuchi M, Nakajima N et al. WO Patent 10
178, 1992-06-25.
[2] Jean-Noel T. FR Patent 2 649 692, 1992-08-03.
[3] Liu Jianrui, Hou Yudong, Gao Shengli et al. Acta Chimica Sinica, 1999, 57: 485-490.
[4] Marthala V K. J. Res. Nat. Bur. Stand., 1980, 85 (6), 467-481.
[5] Liu Jianrui, Yang Xuwu, Hou Yudong et al. Thermochimica Acta, 1999, 329: 123-127.