http://www.chemistrymag.org/cji/2000/02a047pe.htm |
Oct. 1, 2000 Vol.2 No.10 P.47 Copyright |
Liu
Changlin, Fu Weiyu, Xu Huibi, Jin Xionglin#, Mao Xi'an##
(Department of Chemistry, Huazhong University of Science and Technology, Wuhan
430074; #Department of Chemistry, Peking University, Beijing 100871; ##Wuhan
Institute of Physics, Academia Sinica, Wuhan 430071, China )
Received Mar. 8, 2000. We are grateful the National Natural Science Foundation of China for the financial support (Grant No. 29871011). The CCDC code of crystallographic data is CCDC132955.
Abstract A nickel(II) macrocycle dimer
[(NiL)2I](I3)3 (where L =
5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene) formed through a NiII-I-NiII
linkage has been synthesized and characterized. The two macrocyclic planes are arranged
parallel to each other, and the whole complex has D4d symmetry. There
seems to be only a weak covalent interaction between the NiII cation and the
iodide anion in the metal macrocycle dimer. This implies that the NiII cations
serve not only as the central metal atoms of the macrocyclic complexes, but also as
receptors for the iodide ion in the sandwich complex. The dependence of u.v.-vis
absorption of [(NiL)2I](I3)3 on temperature indicates
that the decomposition of the complex may occur at about 375K. The macrocyclic dimer is a
potential electrolyte in the photoelectrochemical cell for converting solar light into
electrical energy, since it contains both iodide and triiodide anions.
Keywords nickel(II) macrocycle, macrocyclic dimer, X-ray crystallography
In the last two decades the synthesis of
macrocyclic ligands and their complexes has been an active area of research [1],
because the complexes are important as potential catalysts [2] and as models
for several metalloproteins [3]. The structures and properties of bi- and
poly-nuclear nickel(II) complexes with various bridging ligands have for many years
received considerable attention. For example, one-dimensional nickel(II) complexes with
bridging ligands such as carboxylato derivatives [4], oxolato derivatives [5],
nitrido [6], halides [7], cyanides [8], thiocyanato
ligands [9], and 1,3-diaminopropane [10] have been synthesized and
well characterized by both X-ray crystallography and spectroscopic methods. At the same
time, a variety of metal macropolycyclic structures such as the macrobicyclic ligands
formed by the coaxial arrangement of two tripod subunits [11], macrotricyclic
ligands produced by bridging two macrocycles [12,13], and macrocyclic dimers in
which two macrocyclic subunits are linked together by one bridge [14 -16], have
also been prepared. The bimetallic complex [Ni(diamine)2]3[Fe(CN)6]X2
(X = PF6-, ClO4-) exhibits a three-dimensional
network extended through a NiII-I-FeII linkage [17].
More recently, particular attention has been paid to the preparation of
complexes containing two transition metal ions separated by a distance of 3.5-7.0 Å to
enhance their ability to form binuclear complexes, but without bridging ligands [18].
The presence of two metal ions separated by a suitable distance defines a cavity where the
coordination of small molecules (O2, N2, CO, etc) and/or organic
substrates can favor oxidation or other reactions. Many such binuclear complexes have been
prepared in order to mimic the reactivity of molecular oxygen and carbon monoxide with
hemocyanin [19], and to study metal-metal interactions, electron transfer and
magnetic properties [20].
The synthesis and characterization of a nickel(II) macrocycle dimer
[(NiL)2I](I3)3 (where L =
5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene), in which two
nickel(II) macrocycles are linked only by the weakly covalent interaction between
nickel(II) and the iodide ion, have been reported in this paper. This complex is a
sandwich one with the iodide as the central atom. This nickel(II) macrocycles can act as a
receptor that selectively accepts the I- anion.
The macrocyclic ligand, L.2HI, and its NiII complex monomer, [NiL]I2, was synthesized according to the well-known procedures [21]. IR (in KBr): 3180(N-H), 1665(imine) cm-1.
1.1 Nickel(II) macrocycle dimer bridged by iodide ion, [(NiL)2I](I2)3.
The macrocyclic ligand (0.05mol) was added to a solution of NiCl2.6H2O (0.05mol) in MeOH (40cm3). The mixture was heated under reflux for 2 h, and the resulting violet solution was set aside to cool at room temperature. Violet crystals of [(NiL)2I](I2)3 in almost quantitative yield were obtained by slow evaporation of the methanol solution of the complex or by cooling the solution in an ice bath. Yield: >85%. Found: C, 20.7; H, 3.5; N, 5.8; I, 65.0; Ni, 6.1%. C32H64I10Ni2N8 calcd.: C, 19.7; H, 3.3; N, 5.8; I, 65.2; Ni, 6.0%.
1.2 Spectroscopic methods.
U.v.-vis absorption spectra of the nickel(II) macrocycle dimer and monomer were obtained using 10-3-10-4 mol.L-1 solution in MeOH or DMF (temperature-dependent experiments) on a Shimazu UV-240 spectrophotometer using 1cm silica cell. IR spectra (KBr discs) were recorded on a Shimazu IR-435 spectrophotometer in the 4000-400cm-1 range. One- and two-dimensional n.m.r. 1H-1H NOESY (nuclear Overhauser effect spectroscopy) and 1H-13C HMQC (heteronuclear multiple quantum coherences) experiments were performed in CD3COCD3 solution on a Bruker AMX500MHz n.m.r. spectrometer at room temperature. The chemical shifts are given in ppm downfield from the 1H signal of TMS.
1.3 Crystal data collection and structure refinement.
The X-ray data for the single crystal of [(NiL)2I](I2)3 was collected on a Rigaku AFC6S four-circle diffractometer using MoKα radiation (m= 0.71069 Å); crystal size: 0.50×0.25×0.08mm. The crystallographic data and some structure refinement parameters are listed in Table 1. The structures were solved by direct methods and refined by full-matrix least-squares methods on F2 using SHELXS-97 program. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located on calculated positions and assigned with isotropic displacement parameters. The final R factor was 0.0507 for all observed reflections [I > 2σ(I)] for 484 variable parameters. Maximum and minimum peaks in the final difference Fourier synthesis were 2.167 and 1.912 e|Å|-3 located near the iodine atoms. The selected bond lengths and angles are reported in Table 2.
Table 1.
Crystallographic Data for the [(NiL)2I(I3)]3
a R(Fo)= å ‖Fo ê - ê Fc‖/ å ê Fo êbwR2(Fo)={ å w[(Fo)2-(Fc)2]2/å w(Fo)2}1/2 |
Table 2 Selected
Bond Distances (Å) and Bond Angles (deg.) for [(NiL)2I](I3)3
|
2 RESULTS AND DISCUSSION
2.1 Synthesis of [(NiL)2I](I3)3
The proposed steps for the formation of the macrocyclic ligand and its complex
with Ni2+ have been given elsewhere [21]. It was reported that the
final product was the nickel(II) macrocycle monomer, [NiL]I2, when the ligand
was reacted with nickel(II) acetate [21]. The generation of the nickel(II)
macrocycle containing this type of protonated ligand depends upon the fact that the
acetate ion is the conjugated base of a weak acid which deprotonates the macrocyclic
ligand, allowing the metal complex to form. An iodide ion then enters the complex since
the anion is a better ligand than the acetate ion. However, we have obtained a new
compound, [(NiL)2I](I3)3, a nickel(II) macrocyclic dimer
formed through NiII-I-NiII linkage, when nickel(II) chloride
hexahydrate was used as the starting material. The chloride ion cannot promote
deprotonation of the macrocyclic ligand because the anion is not conjugated base of a weak
acid. On the other hand, the chloride ion is a better ligand than the iodide ion based on
the their properties of coordination to transition metals. Thus, the chloride ion ought to
bind to the nickel ion as a ligand, but the fact is that we have not found any detectable
amount of complex that contains a chloride ion. In addition, the addition of nickel(II)
bromide does not result in the formation of a bromide analogue to [(NiL)2I](I3)3.
This result suggests that (NiL)24+ is a specific receptor which can
selectively recognize I-. In the metal macrocycle dimer, the triiodide ions are
produced by reaction of the iodide ion with I2 due to substantially higher
stability of I3- in non-aqueous media such as methanol which was
utilized as the solvent. Therefore, we suppose that the formation of the triiodide ion is
attributed to the transportation of the free I2 in hydrogen iodide and the I2
formed by the decomposition of the weak acid from the mixture solution of the ligand
synthesis to the solution of the nickel(II) complex synthesis by the macrocyclic ligand.

Fig1. ORTEP representation (30% of probability
level)of two independent nickel(II) macrocycle subunits.
2.2 Crystal structure of [(NiL)2I](I3)3
In order to understand the structure of the nickel(II) macrocycle dimer bridged by the
iodide ion, and the location of the iodide ion in the complex, its crystal structure has
been determined. In Table 2 the selected bond lengths and angles relevant to the nickel
coordination sphere are reported. An ORTEP representation of two centrosymmeteric
nickel(II) macrocycle units and unit cell packing of the dimer are shown in Figs. 1 and 2,
respectively. Fig. 2 clearly shows that the two macrocycles in [(NiL)2I](I3)3
are staggered. The two independent [(NiL)2I](I3)3
complexes are nearly isostructural (Table2). The distance between the two nickel atoms is
6.32Å, i.e., the Ni(1)-I(1) and Ni(2)-I(2) distances are 3.16Å, whereas for nickel(II)
complexes in which the iodide ions serve as the ligands the metal-iodine bond length is
2.8-2.9 Ao. Furthermore, this metal-metal distance is longer than those
distances found for nickel bimacrocycles linked by various bridging ligands or by various
organic groups between macrocyclic ligands [6,16]. The observed Ni-I distance
indicates that only weakly covalent bond may be formed between Ni2+ and I-.
Ionic bonds cannot be formed between these ions. Therefore, we suppose that the nickel
ions serve not only as the central metal atoms of the two macrocyclic ligands, but also as
receptors for to the iodide ions in the sandwich compound. In other words, the nickel(II)
macrocycle moiety can act as the receptor for the anion.
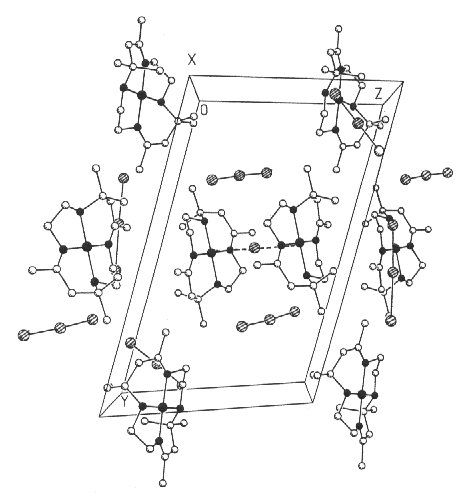
Fig 2. Unit cell packing of the nickel(II) macrocycle dimer
[(NiL)2I](I3)3 showing the interaction between the metal
macrocycle and the iodide ion, and the location of the triiodide ions.
In the crystal, each nickel(II) macrocyclic
monomer is essentially square planar. Each nickel atom in [(NiL)2I](I3)3
lies above the mean plane of the bonded nitrogen atoms by only 0.022Å, and
N(11)-Ni(1)-N(13) and N(12)-Ni(1)-N(14), N(21)-Ni(2)-N(23) and N(22)-Ni(2)-N(24) bond
angles are equal to 176.2(3)o and 178.3(3)o, 178.3(3)o
and 176.8(3)o, respectively. The Ni(1)-N and Ni(2)-N bond distances range from
1.896(8) to 1.939(8), and 1.900(8) to 1.930(8) Å, respectively, and lie in the range of
those found for nickel(II) complexes with tetraazacyclodentate ligands [22].
The bond length differences between Ni(1)-N(11)[1.905(8)] and Ni(1)-N(13)[1.896(8)],
Ni(2)-N(21)[1.900(8)] and Ni(2)-N(2)[1.902(7)] are not significant. These bond distances
are shorter than the corresponding values for Ni(1)-N(1)[1.939(8)] and
Ni(1)-N(14)[1.927(8)], Ni(2)-N(22)[1.929(8)] and Ni(2)-N(24)[1.930(9)]. The difference is
attributed to the differing nature of the amine groups: atoms N(11), N(13), N(21) and
N(23) belong to secondary amines, and the other four nitrogen atoms belong to tertiary
amines. This situation is contrary to dinickel(II) complexes with cylindrical macrocyclic
ligands [16]. It is noteworthy that the Ni(1)-N(12) bond distance is longer
than Ni1-N14 bond in one of the nickel(II) macrocycle subunits, but that these two values
are nearly equal in another subunit.
The chelating angles subtended by the nitrogen donors at the central
atom show deviations from 90o, 87.5(3)o and 87.2(3)o,
86.7(3)o and 86.7(3)o for the five-membered rings are smaller than
92.4(3)o and 93.0(3)o, 93.4(3)o and 93.3(3)o
for six-membered rings. These angles are normal for planar tetraamine 14-membered
macrocyclic ligand complexes of nickel(II) with alternating five-and six-membered chelate
rings [23].
The three triiodide ions in [(NiL)2I](I3)3 are basically linear with bond angles 177.624)-178.69(4)o. Two I3- anions are oriented parallel to the Ni-I-Ni axis and perpendicular to each nickel(II) macrocyclic plane. In solution, the triiodide ion appears to be linear and symmetrical, as in Ph4As+I3-; but in crystalline environments it is often unsymmetrical, though consistently linear or nearly so. In most cases where it does not reside on a crystallographic center of symmetry, the I3- anion is distinctly unsymmetrical; the two I-I distances may differ by as little as 0.06Å or by as much as 0.20 to 0.33Å [22]. Nevertheless, the very small differences of 0.006-0.08Å between two I-I bond distances in the nickel(II) macrocycle dimer demonstrate that the large nickel macrocycle dimer cation favors the more compact I3- anions, and the I3- anions have the greater tendency to be symmetrical.
2.3 Temperature dependent absorption spectra of [(NiL)2I](I3)3
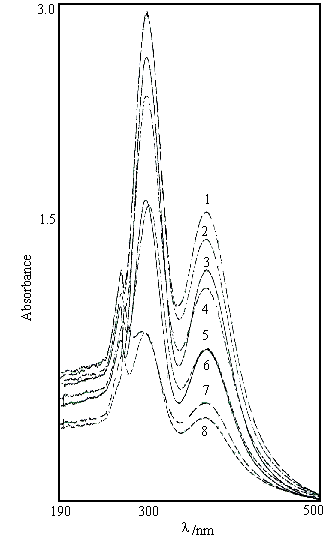
U.v-vis absorption spectroscopic data for the [(NiL)2I](I3)3 and [NiL]I2 complexes are listed in Figure 3 Table3. These data indicate that the absorption properties of the iodide-bridged nickel(II) macrocycle dimer, are remarkably different from the nickel(II) macrocycle monomer. The most obvious absorption difference between [(NiL)2I](I3)3 and [NiL]I2 in MeOH or DMF solution is the metal d-d transition band. The low wavelength absorption band (358 nm) in[(NiL)2I](I3)3 can be ascribed to the presence of iodide ion which may raise the orbital dx2-y2 in energy or lower the other four d orbitals. On the other hand, the ligand-metal charge transfer (LMCT) band of the dimer is red-shifted from 216 nm in methanol to 254 nm in DMF, possibly due to a solvent effect. The two absorption bands have been only observed for [NiL]I2 in DMF.
Table3 U.v-vis absorption spectroscopic data for the [(NiL)2I](I3)3 and [NiL]I2 complexes
Complex Solvent l max(nm) e (M-1cm-) [(NiL)2I](I3)3
CH3OH(298K)
216sha
2.1×104 358 7.5×103 DMFb(298K) 254 2.1×103 285 1.3×104 357 4.6×103 DMF(357K) 261shc 1.3×104 357 170 [NiL]I2 DMF(298K) 256sh 1.6×103 357 190 |
aA strong shoulder to the
high energy side.
bDMF, N,N-dimethyl formamide.
cA weak shoulder to the high energy side.
Fig.3 shows the dependence
of UV-visible absorption of the complex on temperature. Thermochromic phenomenon
frequently encountered among Ni2+ complexes arises from the
temperature-dependent variability of structure, which causes variation in the d-d
absorption bands. However, the temperature-dependent change of structure in [(NiL)2I](I3)3
causes a variation in the LMCT band and charge transfer transition band of the π electrons of the imine
group. The intensities of the three bands decrease with the increase of temperature below
348 K. When the temperature is in the range of 348-375 K, the varying trend of intensity
is contrary to that below 348 K. These three absorption bands present a remarkable
transformation point when the temperature is about. 375 K. Above the temperature, the π electron transition band
of the macrocyclic ligand vanishes from a shoulder, and LMCT band changes from weak to
strong with a small red shift. Finally, the absorption spectrum is basically identical to
that of [NiL]I2 in DMF solution. These results show that the nickel(II) macrocycle dimer appears to decompose into two metal macrocycle
monomers at 375K, i.e., the dimer bridged by the iodide ion may be stable only below 375K.
In addition, the structural alteration in [(NiL)2I](I3)3
is irreversible, in contrast to the (NRxH4-x)2NiCl4
compounds with reversible thermochromism [22].
2.4 One- and two-dimensional n.m.r. of [(NiL)2I](I3)3
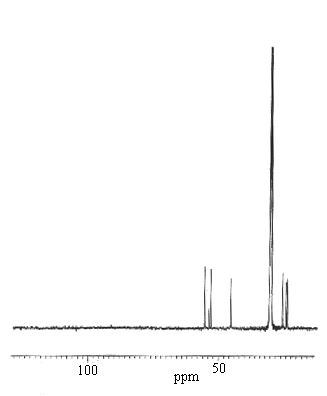

Fig.4. The 1H (right) and 13C (left) n.m.r. spectra of
the complex [(NiL)2I](I3)3
The one- and two-dimensional n.m.r. 1H-1H NOESY and 1H-13C HMQC spectra and data obtained in CD3COCD3, shown in Fig.4 and Table 4, respectively, indicate clearly that all protons and 13C nuclei are observable, and suggest that the dimer may be diamagnetic as in [NiL]I2. In the 1H spectrum (Fig.4, left), the three non-equavalent methyl groups (carbon 4,7,8) appear as sharp singlets at 2.21, 2.05, and 1.26 ppm downfield from TMS. The four dimethylene protons (carbon 1,2), appear at 3.69, 3.53, 2.86, and 2.68 ppm, respectively, and are broadened by the quadruple moment of the nitrogen atoms. The methylene proton resonance at carbon 5 appears as a sharp and strong peak at 3.01 ppm near the residual water resonance (3.12 ppm). The protons of the two N-H groups are located at 5.15ppm. In the 13C spectrum (Fig.4, right), there are 8 peaks which can be easily assigned. Methyl carbons are 23.4, 23.9, and 25.2 ppm, respectively, and metylene carbons are 45.2, 52.8, and 53.5ppm, respectively. In addition, the resonances for carbon three and six appear at 183.3 and 55.3 ppm, respectively. It is unfortunate that we were unable to distinguish between the stereo-arrangement of one nickel macrocycle and another based on these 1H and 13C n.m.r. signals, due to the fast rotation or exchange of the monomeric complexes. That is, the n.m.r. data could not unambguously indicate whether the solution structure of [(NiL)2I](I3)3 was identical to its crystal structure or not.
Table4 1H and 13C Chemical Shifts(ppm) in the complex [(NiL)2I](I3)3
| Carbon | 1H | 13C | |
| 1 | 2.86+2.68(1)a | 45.2 | 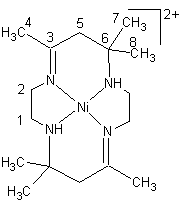 |
| 2 | 3.69+3.53(2) | 53.5 | |
| 3 | 183.4 | ||
| 4 | 2.21 (2,3) | 25.2 | |
| 5 | 3.01 (3,4) | 52.8 | |
| 6 | 55.3 | ||
| 7/8 | 1.35+2.05(1,4) |
23.9/23.4 |
The numbers in all () indicate that a NOE occur between those two groups designated by the same number.
3 CONCLUSIONWe have synthesized and characterized a novel centrosymmetric nickel(II) macrocycle dimer linked by the iodide ion. The dependence of u.v-vis absorption of [(NiL)2I](I3)3 on temperature indicates that the complex may decompose at about 375K. The macrocyclic dimer is a potential electrolyte in the photoelectrochemical cell, since it contains both iodide and triiodide anions. REFERENCES
[1] Lindoy L F. Chemistry of Macrocyclic Ligand and Complexes. Cambridge: Cambridge University Press, 1989.
[2] McKenzie C J, Robson R. J. Chem. Soc., Chem. Commun., 1988, 112
[3] Fenton D E. in Sykes A G (Ed.). Advances in Inorganic and Bioinorganic Mechanisms(II). London: Academic Press, 1983, 187.
[4] Coronado E, Drillon M, Fuertus A et al. J. Am. Chem. Soc., 1986, 108: 900.
[5] Soules R, Dahan F, Laurent J P et al. J. Chem. Soc. Dalton Trans., 1988, 587.
[6] Meyer A, Gleizes A, Girerd J J et al. Inorg. Chem., 1982, 21: 1729.
[7] Kouche-Waksman I, Haridom P L. Bull. Soc. Chim. Fr., 1983, 25: 30.
[8] Hasegawa T, Nishikiori S, Iwamoto T. Chem Lett., 1985, 1659.
[9] Turpeinen U, Ahlgren M. Finn. Chem. Lett., 1977, 75: 274.
[10] Anbanandam A, Babu V, Andrea C et al. Inorg. Chem., 1998, 37: 228.
[11] Lehn J M. Acc. Chem. Res., 1978, 11: 49.
[12] Kotzyba-Hibert F, Lehn J M, Saigo K. J. Am. Chem. Soc., 1981, 103: 4266.
[13] McAuley A, Subramanian S. Inorg. Chem., 1997, 36: 5376.
[14] Zhang X, Hsieh Y W, Margulis T N et al. Inorg. Chem., 1995, 34: 2883.
[15] Alberts A H, Annunziata R, Lehn J M. J. Am. Chem. Soc., 1977, 99: 8502.
[16] Mohamme L, Roger G, Aziz A et al. Inorg. Chem., 1998, 37: 1575.
[17] Fokita N, Ohba M, Okawa H et al. Inorg. Chem., 1998, 37: 842.
[18] Guerriero P, Tamburini S, Vigato P A. Coord. Chem. Rev., 1995, 139: 17.
[19] Pyrz J M, Karlin K D, Sorrel T N et al. Inorg. Chem., 1984, 23: 4581.
[20] Karlin K D, Zubieta J (Ed.). Copper Coordination Chemistry: Biochemical and Inorganic Perspectives. Guilderland, NY: Adenine Press, 1984.
[21] Merrell P H, Urban F L, Arnold M. J. Chem. Educ., 1977, 54: 580.
[22] Cotton F A, Wilkinson G. Advanced Inorganic Chemistry. 5th edn. Wiley, NY: Wiley-Interscience, 1988, 577.
[23] Barefield L, Freeman G M, Van Derveer D G. Inorg. Chem., 1986, 25: 552.