http://www.chemistrymag.org/cji/2001/032009ne.htm |
Feb. 21, 2001 Vol.3 No.2 P.9 Copyright |
Sun Jing, Jiang Yanming, Yan Chaoguo
(Department of Chemistry, Yangzhou University, Yangzhou 225002)
Received Jun. 15, 2000.
Abstract With potassium fluoride
supported on alumina as solid catalyst, rhodanine 1 (2-thioxo-4-thiazolidinone)
reacted with aromatic aldehydes 2a-e in refluxing methanol to give
5-arylmethylenerdonines 3a-e in high yields. The UV spectra of 3a-e in
seventeen solvents were measured, and the maximum absorption frequency shows good
correlation with the Hammett's substituent cofficients.
Keywords rhodanine, catalyzed synthesis, UV spectra, substituent effect.
The interesting relationship of the
electronic spectra with the structure of organic molecules as well as with the environment
has been attracted more and more attention. Although there are a lot of qualitative and
quantitative theory and rules in this fields, the limitation and drawback were disturbed
in application[1-3], which can only be solved by more research and data on UV
spectra of different types of organic compounds.
We have synthesized 5-arylmethylenerdonines 3a-e by using KF-Al2O3
as solid base catalyst for the first time and the products were studied by UV
spectroscopy, from which the linear correlation with the Hammett's
substituent parameter was obtained.
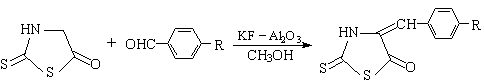 1 2a-e 3a-e |
Table 1 The Data of 5-Arylmethylenerhodanine 3a-e
| Entry | R |
Yield m. P.
(lit. [5] ) |
1 H NMR |
3a |
H |
80 199-202(202-203) |
3.35 (br, 1H,
NH) |
3b |
p-Cl |
87 224-230 (225-226) |
1.61 (br, 1H,
NH) |
3c |
p-CH3 |
83 219-223 |
2.41 (s, 3H,
CH3) |
3d |
p-OCH3 |
82 240-246 (230-242 ) |
3.86 (s, 3H,
CH3) |
3e |
p-N(CH3)2 |
83 266-270 (240-270) |
3.11 (s, 6H,
2CH3) |
The aldol condensation of
rhodanine with aldehydes have been known to take place under the catalysis of weak bases
(such as amine or alkaline acetate)[4-5]. Recently, potassium fluoride
supported on alumina has been found effective solid catalyst and widely used in many
organic reactions, especially the condensation reactions of active methylene compounds[6-8].
Thus, the preparation of 5-arylidenerhodanines catalyzed by KF Al2O3 for
the first time has been reported in this paper. The higher yield was obtained under higher
temperature. If the reaction was carried out in refluxing conditions, the yield is more
than 80% (Table 1). This means that even the relatively inactive benzaldehydes with
electron-donating group such as p-methyl p-methoxyl and p-dimethylamino group as
substituent, the reaction can also go very easily under these conditions. The structures
of the 5-arylmethylenerhodanines were determined by their melting point, IR and 1H
NMR spectra, which were all consistent with that reported in the literature.
Furthermore, the UV spectra of the five 5-arylidenerhodanines in
seventeen solvents were measured. The maximum absorption wavelength and frequency nmaxuv were listed
in table 2.
Table 2 The maxima lmaxuv and nmaxuvof 5-arylmethylerdoniene 3a-e
| solvent | 3a H |
3b p-Cl |
3c p-CH3 |
3d p-OCH3 |
3e p-NMe2 |
|||||
| lmaxuv | nmaxuv | lmaxuv | nmaxuv | lmaxuv | nmaxuv | lmaxuv | nmaxuv | lmaxuv | nmaxuv | |
| cyclohexane | 365.9 |
2.7330 |
385.7 |
2.5927 |
387.5 |
2.5806 |
400.9 |
2.4944 |
454.5 |
2.2002 |
| dichloromethane | 380.9 |
2.6254 |
384.5 |
2.6008 |
387.9 |
2.5780 |
398.5 |
2.5094 |
472.5 |
2.1164 |
| chloroform | 380.1 |
2.6309 |
383.3 |
2.6089 |
385.5 |
2.5940 |
397.5 |
2.5157 |
473.7 |
2.1110 |
| Carbon tetrachloride | 379.1 |
2.6378 |
383.5 |
2.6076 |
385.7 |
2.5927 |
399.1 |
2.5056 |
457.9 |
2.1839 |
| dichloroethane | 379.5 |
2.6350 |
383.3 |
2.6089 |
385.7 |
2.5927 |
396.5 |
2.5221 |
470.7 |
2.1245 |
| benzene | 381.3 |
2.6126 |
386.3 |
2.5887 |
387.9 |
2.5780 |
400.1 |
2.4994 |
463.1 |
2.1594 |
| toluene | 381.1 |
2.6240 |
385.7 |
2.5926 |
387.5 |
2.5806 |
398.3 |
2.5107 |
460.7 |
2.1706 |
| methanol | 373.5 |
2.6714 |
376.3 |
2.6575 |
378.9 |
2.6392 |
397.9 |
2.5132 |
457.5 |
2.1858 |
| ethanol | 373.9 |
2.6745 |
377.7 |
2.6476 |
380.1 |
2.6309 |
391.3 |
2.5569 |
445.3 |
2.2457 |
| 2-propanol | 372.5 |
2.6846 |
375.9 |
2.6603 |
378.7 |
2.6406 |
389.7 |
2.5661 |
458.3 |
2.1800 |
| 1-butanol | 382.9 |
2.6116 |
378.1 |
2.6418 |
381.3 |
2.6226 |
399.5 |
2.5031 |
478.3 |
2.0907 |
| 2-methyl-1-propanol | 372.3 |
2.6860 |
376.3 |
2.6575 |
379.7 |
2.6336 |
391.5 |
2.5547 |
459.9 |
2.1739 |
| ether | 374.5 |
2.6702 |
387.7 |
2.5793 |
380.9 |
2.6254 |
392.3 |
2.5491 |
448.3 |
2.2316 |
| 1,4-dioxane | 375.9 |
2.6603 |
378.7 |
2.6337 |
382.5 |
2.6144 |
391.3 |
2.5543 |
453.5 |
2.2051 |
| acetonitrile | 373.9 |
2.6745 |
377.9 |
2.6462 |
380.1 |
2.6309 |
388.7 |
2.5727 |
465.3 |
2.1491 |
DMF |
373.5 |
2.6774 |
377.9 |
2.6462 |
377.9 |
2.6462 |
385.3 |
2.5934 |
426.3 |
2.3458 |
DMSO |
380.3 |
2.6295 |
383.1 |
2.6103 |
385.7 |
2.5927 |
392.7 |
2.5465 |
472.1 |
2.1182 |
It can be seen that the all of substituted groups on the benzene ring can cause the maximum absorption shift bathochromic. It has been well known that the complementary electronic transitions need lower energies no matter the substituent withdrawing or accepting electrons. In order to esteemed the quantitative effect of each substituted group on the UV spectra, we tentatively correlated the maximum wavelength with Hammett's substituent parameter[9](s+p for H, Cl, CH3, OCH3, (CH3)2N is 0, 0.11, -0.31, -0.78, -1.7, respectively). The fine results from the correlation equation nmaxuv= A + B|s+p| was observed. 11 correlation cofficients, R, in 17 were above 0.97, 4 between 0.97 and 0.96, 2 between 0.95 and 0.96 (shown in table 3). The results show that the conjugative effects play more important roles [10 ].
Table 3 Correation of UV Data with Hammett's s+p
| solvent | A |
B |
R |
cyclohexane |
2.68212 |
-0.27921 |
-0.97679 |
dichloromethane |
2.65745 |
-0.2956 |
-0.97139 |
chloroform |
2.6684 |
-0.30397 |
-0.96978 |
Carbon tetrachloride |
2.65974 |
-0.26418 |
-0.98316 |
1,2-dichloroethane |
2.66861 |
-0.2965 |
-0.96924 |
benzene |
2.64121 |
-0.26481 |
-0.97533 |
toluene |
2.64758 |
-0.26187 |
-0.97451 |
methanol |
2.70452 |
-0.29293 |
-0.98982 |
ethanol |
2.69558 |
-0.24907 |
-0.98042 |
2-propanol |
2.71648 |
-0.29269 |
-0.97309 |
1-butanol |
2.68149 |
-0.32333 |
-0.96749 |
2-methyl-1-propanol |
2.7136 |
-0.29334 |
-0.9774 |
ether |
2.66994 |
-0.23934 |
-0.95616 |
1,4-dioxane |
2.6862 |
-0.26307 |
-0.97186 |
acetonitrile |
2.7106 |
-0.3033 |
-0.96187 |
DMF |
2.69183 |
-0.1897 |
-0.9723 |
DMSO |
2.67131 |
-0.29632 |
-0.95456 |
Rhodanine and aromatic aldehydes are commercial reagents for organic
synthesis. Solid base KF-Al2O3 (12.5mmol KF on 2.00g alumina) was
prepared according to the literature methods[7]. Absolute methanol and the
solvent for determining UV spectra are analytical reagents, which were treated according
to the standard methods before use. Melting points were determined by capillary tube
method. IR spectra were recorded as KBr disks on a Nicolet 170SX IR Spectrometer. 1H
NMR data were determined on a Bruker spectrometer(300MHz) with CDCl3 as solvent
and TMS as internal reference. UV spectra were recorded on UV 240 spectrometer.
General Procedure for the reaction of Rhodanine with aromatic
aldehydes: To a solution of rhodanine ( 1.33g, 10.0 mmol) and aromatic aldehyde (10.5
mmol) in methanol (20 mL), KF-Al2O3 (1.50g, 9.0 mmol) was added.
After stirring under refluxing for about 4 hours, the solid catalyst was filtered off from
the reaction mixture. Then the solution was cooled down to room temperature and poured
into water. The yellow precipitate was collected and the pure products were obtained by
recrystallization from the mixture of ethanol and water.
REFERENCE
[1] Kamlet M J, Abboud J S, Taft R W. J. Amer. Chem. Soc., 1977, 99: 6027.
[2] Reichardt C, Angew. Chem. Int. End. Engl., 1979, 18: 98.
[3] Wang C S, Pan J X, Gao Zh H. Acta Chimica Sinica (Huaxue Xuebao) , 1988, 46: 548.
[4] Girard M L, Dreux C. Bull. Soc. Chim. Fr., 1968, 8: 3461.
[5] Ginak A Z, V yanov K A, Sochilin E G. Zh. Org. Khim., 1971, 7: 1500.
[6] Clark J H. Chem. Rev., 1980, 80 (5): 429.
[7] Yamawaki J, Kawat T, Ando T et al. Bull. Soc. Japan, 1983, 56 (6): 1885.
[8] Lu W X, Zhu Q,Yan C G. Synthetic Commun., 1997, 27 (22): 3985.
[9] March J. Advanced Organic Chemistry (2nd edition), New York: McGraw-Hill Book Company,
253.
[10] Gao Z H, Zhao A H, Wang M Z. Chem. J. of Chin. Univ, 1989, 10 (5): 477.