http://www.chemistrymag.org/cji/2001/032010pe.htm |
Feb. 21, 2001 Vol.3 No.2 P.10 Copyright |
Synthesis and crystal structure of a polymer assembled by non-covalent weak interactions
Chen Zilu, Liang Fupei, Hu Ruixiang, Liang Hong, Yu Kaibei #
(Department of Chemistry, Guangxi Normal University, Guilin 541004, China; #Analysis
Center, Chengdu Branch of Chinese Academy of Science, Chengdu 610041, China)
Abstract Treatment of Y(pic)3
with 1, 10'-phenanthroline and 4, 4'-bipyridine in acidic environments yields a kind of
polymer assembled by non-covalent weak interactions. It was characterized by single
crystal X-ray diffraction which shows that a novel supramolecular unit of [(phenH)(4,
4'-bpyH)(phen)](pic)2 ( 4, 4'-bpy: 4, 4'-bipyridine, phen: 1,
10'-phenanthroline, pic-: picric anion ) was formed via hydrogen bonds among
one 4, 4'-bipyridine molecule and two 1, 10'-phenanthroline molecules and p-p stacking effect between
pyridyl ring from 4, 4'-bpy and phenyl ring from picric anions, then a 2D brick-wall
structure is constructed by p-p stacking effect among different units in the form of head-to-tail
overlap.
Keywords picric anion, phen, 4, 4'-bpy, hydrogen bond, p-p stacking effect
1. INTRODUCTION
All chemists are familiar with intramolecular covalent bonds, but less familiar with
the concepts of intermolecular non-covalent bonds between molecules and/or ions[1].
Indeed, the non-covalent interactions such as p-p stacking effect, hydrogen bonds and host-guest interactions play
an important role in the formation of supramolecules and crystalline form. So the nature
of non-covalent bond gets to the center of interest in organic or inorganic chemistry and
leads to the rapid development in this new field.[2-7] A series of concepts
such as self-assembly, supramolecular synthesis and crystal engineering are followed hand
at heel.[8] This provides reliable theoretical basis and practical synthetic
routes for the design of new type functional materials demonstrating special characters
such as non-linear optical property, superconductivity and ferro-magnetism.[5-6]
4, 4'-Bipyridine is an excellent rigid bridging ligand and liable to
have some weak intermolecular interactions such as hydrogen bonding and p-p stacking effect with
other molecules. Many structures have been done on the coordination of 4, 4-bipyridine to
metals, but less done on the non-covalent weak interactions of 4, 4-bipyridine with other
molecules. We have synthesized a polymer assembled non-covalent weak interactions and
found that 4, 4'-bipyridine has other two kinds of non-covalent bridging modes ( hydrogen
bridging mode, p-p
stacking bridging mode ), which have been
reported in the literature.
2.1 Materials and methods
All starting chemical reagents were commercially available with analytic grade except that 4, 4'-bipyridine with reagent grade. Rare earth picrate was prepared according to a literature method[9]. Elemental analyses ( C, H, N ) were done on a German EL-CHNS-O elemental analyser. Infrared spectrum was measured with American Nicolet 5DXB FT-IR spectrophotometer on KBr disk.
2.2 Synthesis of the complex
Y(pic)3·5H2O ( 0.5 mmol, 0.4319g ) was dissolved in CH3CN-H2O ( 1:1 V/V, 10 ml ) at 50°C, then CH3CN-H2O (1:1 V/V, 10 ml ) solution containing phen ( 1 mmol, 0.1982g ) and CH3CN-H2O (1:1V/V, 10 ml) solution containing 4, 4'-bipyridine (0.5 mmol, 0.0781g ) were added stepwise with stirring, yielding a small amount of precipitate. 20h later, the reaction mixture was filtered and the filtrate was laid aside to evaporate for one month, yellow needle crystals were obtained. A single crystal suitable for X-ray analysis was obtained by redissolving the yellow needle crystals in absolute ethanol and evaporating the solvent at room temperature for one month. Anal. Found: C, 56.68%; H, 3.10%; N, 17.24%. Calc. for C46H30N12O14: C, 56.77%; H,3.07%; N,17.28%.
2.3 Crystallography
Crystal data were collected on Siemens P4 diffractometer using graphite-monochromated Mo Ka radiation ( l = 0.071073 nm ). The intensities of reflections were measured in the w scanning mode at 296(2) K. Cell Parameters of the complex were determined from 25 reflections in the q range of 2.15° -25.00° . An empirical absorption correction was applied to the original data set. The structure was solved using direct methods and refined by a full-matrix least-squares on F2 technique using the SHLXL-97 program package. Hydrogen atoms of the ligands were generated geometrically. a = 0.73370(10) nm, b = 1.03390(10) nm, c = 1.5195(2) nm, a= 75.990(10)° , b = 84.780(10)° , g = 70.160(10)°, V = 1.0519(2)nm3, Z = 1, Dc = 1.539 g·cm 3, m = 0.118 mm-1, F(000) = 502, R1 = 0.0467, wR2 = 0.1143, W = 1 / [ s2F02 + ( 0.0685 P )2 ], (D/s)max = 0.000, Drmin = -366 e/nm3, Drmax = 588 e/nm3. 3. RESULTS AND DISCUSSION
3.1 Synthesis of the complex
The reactions among yttrium picrate, 1, 10-phenanthroline and 4, 4'-bipyridine in CH3CN-H2O ( 1:1 ) gave some precipitates. The crystals obtained from the filtrate were found to be soluble in solvents such as CH3CN, CH3COCH3 and CH3CH2OH, but very difficult to dissolve in H2O. Suitable crystals were not easy to obtain for single crystal X-ray diffraction. The result of X-ray diffraction indicates that there are some protonized 4, 4'-bpy existing in the compound. This is caused by the yttrium picrate which was not thoroughly purified. The solution of yttrium picrate in CH3CN-H2O ( 1:1 ) has the pH value of 4. In this acidic environment, some 4, 4'-bipyridine molecules are easily protonized.
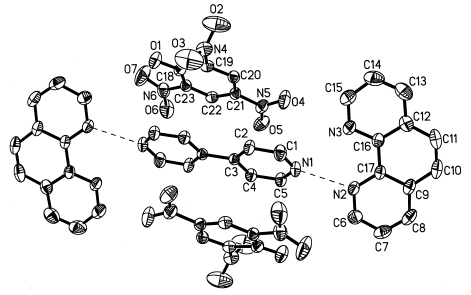
The crystal structure consists of centro-symmetric supramolecular units assembled from 4, 4'-bipyridine, 1, 10'-phenanthroline and picric anions via intermolecular weak interactions. In this supramolecular unit, 4, 4'-bpy molecule acts as two kinds of weak bridging function. One is hydrogen bonding bridge , that is to say, one 4, 4'-bpy molecule bridges two 1, 10-phenanthroline molecules via H-bond of the type of N-H ... N ( N1 ... N2: 0.2716 nm, N1-H1N ... N2: 164(5)°, N2-H2N ... N1: 167(5)° ). The other is that one 4, 4'-bpy molecule bridges two picric anions via p-p stacking effect between pyridyl ring of the 4, 4'-bpy molecule and phenyl ring of the picric anion. The phenyl rings, which are parallel to the pyridyl rings of the 4, 4'-bpy, overlap the nearest pyridyl rings with the separation of 0.3316nm, indicating significant p-p stacking effect. Two noncoplanar pyridyl rings of the 4, 4'-bpy molecule are parallel to each other with the separation of 0.00279nm, different from the reported cases[10-12] in which two pyridyl rings of the 4, 4'-bpy molecule are coplanar or twisted with a certain dihedral angle. One pyridyl ring of the 4, 4'-bpy molecule and the nearest 1, 10-phenanthroline molecule are non-coplanar with a dihedral angle of 42.6°. The packing of such units in the ac plane is dominated by p-p interactions between phen molecules in the form of head-to-tail overlap with the separation of 0.3561nm, which is comparable to the reported cases[12-14]. So formed a two-dimensional brick-wall structure in the ac plane. Between layers only exist electrostatic force and Van der Waals force.
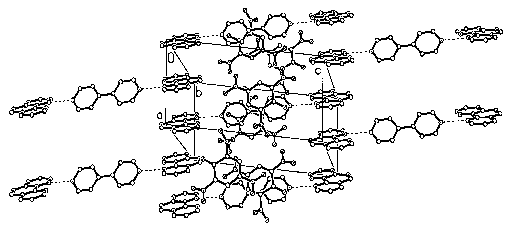 Fig. 2 Two-dimensional brick-wall structure in the ac plane
Fig. 2 Two-dimensional brick-wall structure in the ac plane
According to the above and literature results, four kinds of bridging mode for 4, 4'-bpy are summarized here. One is coordination bridging mode ( M-N(4, 4'-bpy)N-M )[14-15], another two are hydrogen bonding bridge ( M-H2O ... N(4, 4'-bpy)N ... H2O-M )[16] and p-p stacking bridging mode described in this paper. And the fourth is an unsymmetric bridging mode containing coordination bond and hydrogen bond simultaneously ( M-N(4, 4'-bpy)N ... H2O-M )[17].
REFERENCES
[1] Aakeroy C B, Sedden K R. Chem. Soc. Rev., 1993, 397-407.
[2] Lehn J M. Angew. Chem. Int. Ed. Engl., 1988, 27:
89-112.
[3] Bakshi P K, Cameron T S, Knop O. Can. J. Chem.,
1996, 74 (2): 201-220.
[4] Crabtree R H, Eisenstein O, Sini G et al. J.
Organomet. Chem., 1998, 567 (1-2): 7-11.
[5] Hosseini M W, Cian A D. Chem. Commun., 1998,
727-733.
[6] Pedireddi V R, Jones W, Chorlton A P et al.
Tetrahedron Lett., 1998, 39 (30): 5409-5412.
[7] Classens C G, Stoddart J F. J. Phys. Org. Chem.,
1997, 10: 254-272.
[8] Russell V A, Ward M D. Chem. Mater., 1996, 8:
1654-1666.
[9] Chen X M, Tong M L, Luo Y J et al. Aust. J.
Chem., 1996, 49: 835-838.
[10] Tian Y C, Liang Y Q, Ni J Z. Chemical Journal
of Chinese Universities (Gaodeng Xuexiao Huaxue Xuebao), 1988, 9: 113-117.
[11] Li J M, Zeng H Q, Chen J H et al. Chem.
Commun., 1997, 1213-1214.
[12] Tong M L, Ye B H, Cai J W et al. Inorg. Chem.,
1998, 37: 2645-2650.
[13] PAN Z Q, Luo Q H, Long D L et al. Chin. J.
Chem., 2000, 18 (1): 124-127.
[14] Carlucci L, Ciani G, Proserpio D M et al. J.
Chem. Soc., Dalton. Trans., 1997, 1801-1803.
[15] Gable R W, Hoskins B F, Robson R. J. Chem.
Soc., Chem. Commun., 1990, 1677-1678.
[16] Lu J, Paliwala T, Lim S C et al. Inorg. Chem.,
1997, 36, 923-929.
[17] Bukowska-Strzyzewska M, Tosik A. Inorg.
Chem. Acta, 1978, 30, 189-196.