http://www.chemistrymag.org/cji/2001/033011pe.htm |
Mar. 1, 2001 Vol.3 No.3 P.11 Copyright |
Group transfer polymerization of ethyl acrylate with triphenylphosphine and trimethylchlorosilane as initiator
Shen Weiping, Jin Hongmei, Shi Zhijian,
Zhu Yun, Li Bing
(Department of Chemistry, Shanghai University, Shanghai 201800, China)
Abstract Triphenylphosphine
(TPP) and trimethylchlorosilane (TMCS) were used directly to initiate group transfer
polymerization (GTP) of ethyl acrylate in the presence of zinc bromide as catalyst. The
poly(ethyl acrylate) with a terminal triphenylphosphonium group was obtained. In this
polymerization procedure, GTP was conveniently carried out. The studies of GTP kinetics of
ethyl acrylate indicate that the concentration of zinc bromide as catalyst has to be kept
in 12-16mol.% based on the concentration of ethyl acrylate at higher TPP concentration so
as to ensure the characteristics of living polymerization. Whether the termination
reaction becomes major role depends to a large extent on the concentration of zinc
bromide. It has been shown that the major pathway for termination in the GTP is a process
of isomerization from O-silyl bond to C-silyl bond via phosphrous ylid
intermidiate.
Keywords triphenylphosphine, trimethylchlorosilane, group transfer
polymerization
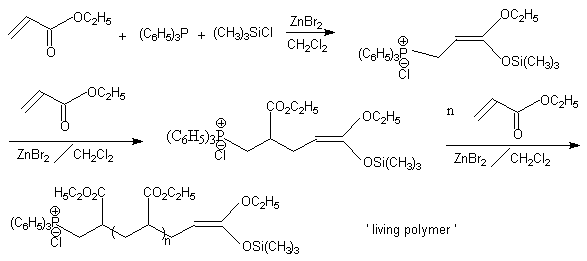
Some new initiators of group transfer polymerization (GTP), 3-methoxy-3-trimethylsiloxy-2- propenyltriphenylphosphonium chloride and its 2-methyl derivatives, were presented firstly in our previous paper [1]. Meanwhile, it was found that GTP of ethyl acrylate(EA) could be conveniently carried out using directly triphenylphosphine(TPP) and trimethylchlorosilane (TMCS) as initiator and zinc bromide (ZnBr2) as catalyst in dichloromethane (Scheme 1). In comparison with dimethylketene methyl trimethylsilyl acetal as initiator[2], this new initiator had some differences : higher concentration of initiator was required and 'living' character of GTP strongly depended on the concentration of catalyst. In this paper, the behavior of this initiator was further investigated.
Scheme 1
1 EXPERIMENTAL
1.1 Materials and methods
Methylene chloride was purified by distillation from P2O5. Ethyl
acrylate was washed with aqueous KOH, then dried over MgSO4 and CaH2,
and distilled under purified argon atmosphere. Before use, they were conserved in CaH2.
Triphenylphosphine was dried at 56°C
i.vac. overnight. Argon was passed over 4 Å molecular sieves. All glassware were dried at
180°C for at least 12 hours .Group
transfer polymerization (GTP) was carried out under purified argon using syringe
techniques for introduction of the liquid reactants and solvents.
1.2 Analyses
NMR spectra were recorded on a Bruker AC-100 SC. 31P NMR dynamic spectra of GTP
of EA (Fig.2) and identification of compounds was detailed at ref.3[3].IR
spectra were recorded on a Perkin-Elmer 580 spectrophotometer. Molecular weights and
polydispersity (D=Mw/Mn) were determined on size-exclusion
chromatography using a Waters Associates 150C ALC/GPC with a 6000 A pump, R 401
differential refractive index detector and two m-Styragel columns, 103 and 104
Å , connected in series. The measurements were made using chloroform as solvent (1
ml/min. at 30°C ), and a
calibration plot was constructed with polystyrene standards(The polystyrene standards are
not ideal ones for polyacrylates, therefore, the molecular weights in Table are apparent
values). The quantitative analysis of double bonds in the time-EA concentration curves was
performed by use of the bromination method[4].
1.3 Polymerization procedure
(example)
A three-necked flask containing 9.2 g (40 mmol) of zinc bromide was heated at 300°C i.vac. for 1 h
and then allowed to cool under purified argon. Then 160 ml of dichloromethane and 36 ml
(338 mmol) of ethyl acrylate was added followed by 20 ml of dichloromethane solution of
triphenylphosphine (6.8 g; 26 mmol) and chlorotrimethyl- silane (3.3 ml;26 mmol) at 0°C. The reaction mixture was stirred for 3h at
0°C,
then 2 ml of methanol was added to quench the reaction. The solvent was partially
evaporated, then ether and hexane were added causing a voluminous precipitate of polymer.
Decantation of the supernatant and removal of the remaining solvent i. vac. gave 40 g of
poly(ethyl acrylate) with terminal triphenylphosphonium group. As revealed by elemental
analysis, it contained 1.77% phosphorous (calc. 1.94%).
IR (KBr): 1730 (s, C=O), 1440 (s, P-Ph) and 1110 cm-1 (s, C-O).
1H NMR (CDCl3): δ=8.05-7.40 (m, Ph), 3.7 (q, OCH2) and 1.2ppm (t, CH3).
GPC: Mn=1726, Mw=1809, D= 1.12 (theoretical Mn=1597).
2 RESULTS AND DISCUSSION
2.1 GTP of EA with TPP and TMCS as initiator
Scheme1 reported triphenylphosphine and trimethylchlorosilane as direct initiators and
zinc bromide as catatyst for group transfer polymerization of ethyl acrylate.
Triphenylphosphonium-containing poly(ethyl acrylate) was obtained. Our results showed that
complete conversions were not obtained for nearly all catalyst concentrations used at
lower initiator concentrations, while, at higher initiator concentrations and catalyst
concentrations of 12-16mol% based on the concentration of ethyl acrylate ([EA]0)
, complete conversions were obtained(see Table). When the catalyst concentration exceeded
20mol% [EA]0, conversions began to decrease and molecular weights of the
polymers were much higher than the theoretical ones and crystalline phosphonium salt was
easily separated from polymerization system which have been identified as addition
products of TPP to EA by 1H NMR measurements. Therefore, as
triphenylphosphonium group was introduced to ketene silyl acetals, it was suggested that
the mechanism in GTP of EA was changed, initiation stage in GTP of EA became complicated
and 'living' centers were terminated easily.
Table 1 GTP of EA initiated directly by TPP and TMCS ([EA]0=1.71mol./l; CH2Cl2; 00C)
[ZnBr2]0 |
Mn |
Mw |
Mw/Mn |
Conv. |
[TPP]0=0.05 mol./l |
||||
5 |
1467 |
1815 |
1.24 |
14.7 |
[ZnBr2]0 |
Mn |
Mw |
Mw/Mn |
Conv. |
[TPP]0=0.10mol./l |
||||
5 |
2256 |
2265 |
1.18 |
24.1 |
[ZnBr2]0 |
Mn |
Mw |
Mw/Mn |
Conv. |
[TPP]0=0.15mol./l |
||||
5 |
1066 |
1478 |
1.39 |
26.2 |
2.2 The kinetics of the GTP of EA
initiated directly by TPP and TMCS
Fig.1 showed time-EA concentration curves for different catalyst concentration[5].
At lower zinc bromide concentration (curve a and b), GTP of EA could not be
carried out using TPP and TMCS directly as initiator. Curve c and d
respectively displayed that the polymerization rate smoothly increased and appeared
self-acceleration behavior similar to radical polymerization. Especially, curve e
indicated that the earlier stage of polymerization was rapid. Then, because of side
reaction, living character of the system appeared 'suspensive' behavior and partial
'living' end-group lost and crystalline phosphonium salts formed. Following GTP still
proceeded at real lower initiator concentration , resulting in polymer with higher
molecular weight. In Fig.1 curve f , side reaction became dominant with increasing
catalyst concentration. Therefore, in this polymerization procedure, a 'living' character
of GTP depended on the catalyst concentration. At higher than 12mol% [EA]0
concentrations of zinc of bromide, the side reaction did not play an important role, but
with increasing catalyst concentration they became more and more dominant.
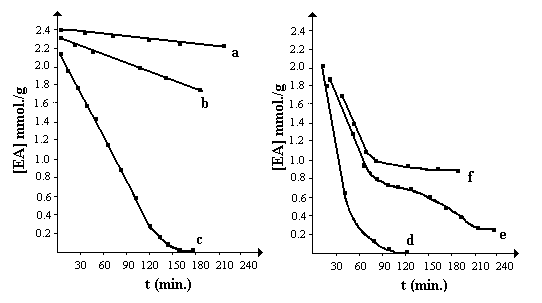
Fig.1 Concentration of EA vs time for GTP
[EA]0=2.88mol./l [TPP]0=0.14mol./l
a:[ZnBr2]0=0.14mol./l(5mol.%[EA]0)
d:[ZnBr2]0=0.46mol./l(16mol.%[EA]0)
b:[ZnBr2]0=0.23mol./l(8mol.%[EA]0)
e:[ZnBr2]0=0.58mol./l(20mol.%[EA]0)
c:[ZnBr2]0=0.35mol./l(12mol.%[EA]0)
f:[ZnBr2]0=0.69mol./l(24mol.%[EA]0)
2.3 The mechanism of the GTP of EA
initiated directly by TPP and TMCS
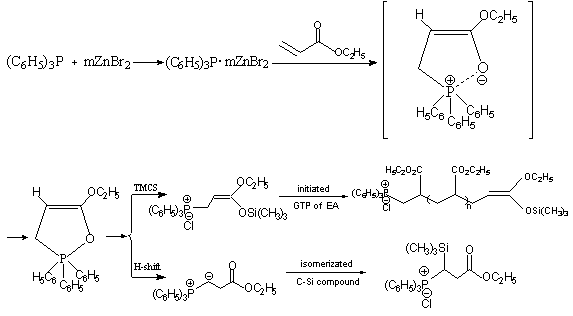
The side reaction caused the termination of GTP of EA and was investigated by 31P
NMR dynamic spectrum[3]. Scheme2 gave the mechanism of the GTP of EA initiated
directly by TPP and TMCS. First, triphenylphosphine coordinated with zinc bromide. Second,
ethyl acrylate was nucleophilly attached by TPP complex. Then, heterocyclic compound
containing P-O bond was formed and further converted to
3-ethoxy-3-trimethylsiloxy-2-propenyltriphenyl-phosphonium chloride in the presence of
TMCS, which initiated GTP of EA and 'living' center was growing. In addition, due to
triphenylphosphonium group was introduced to the system, O-to-C isomerization of ketene
silyl acetal[6] occurred through phosphorous ylid intermediate, which had
arised from initiation process. Initiation step contained conjugated addition reaction of
TPP and TMCS to EA and Micheal addition of triphenyl- phosphonium-containing ketene silyl
acetal to EA so the initiation process would be accompanied with more side reactions[7,8].
The zinc bromide was assumed to coordinate with not only the monomers but the
triphenylphosphine as initiator and made it form phosphorous ylid more easily. In both
initiation and propagation stage intramolecular proton transfer had occurred and
phosphorous ylid was formed resulting in C-silyl compound which is not efficient GTP
initiator and tends to give phosphonium salts, whose moleculer weights are not as well
controlled as in the case of ketene silyl acetals[9]. It is different from
using 1-methoxy-2-methyl-1-(trimethylsilyloxy)-1-propene as initiator which terminated to
form cyclic compound by back bitting reaction[10]. But intramolecular proton
transfer gradually disappeared, as polymeric chain propagated. So the terminated process
was especially evident in the previous procedure of GTP. The side reaction was mainly
accompanied with initiation step of GTP, so that higher concentrations of TPP and TMCS
were needed in this polymerization procedure. At higher concentration of zinc bromide,
active of nucleophilic reaction was greatly increased. Ketene silyl acetal as efficient
initiator for GTP was rapidly formed, resulting in group transfer polymerization
proceeded. At lower concentration of zinc bromide, addition reaction was very slow and GTP
of EA was unsuccessful.
Scheme 2
[1] a) Shen W, Zhu W et al. Makromol. Chem., 1989, 190: 3061 .
b) Shen W, Jin H. Makromol. Chem., 1992, 193: 743.
[2] Hertler W R, Sogah D Y, Webster O W. Macromol., 1984, 17: 1415.
[3] Shi Z, Shen W et al. Chinese Journal of Magnetic Resonance, 1995, 12: 229.
[4] Riddle E H. Monomeric Acrylic Esters, Philadelphia Rohm & Hass Company, 1954.
[5] Xu L, Sen K. Acta Polymeric Sinica, 1990, (5): 628.
[6] Quirk R P, Bidinger G P. Polymer Bulletin, 1989, 22: 63.
[7] Bandermann F, Speikamp H D. Makromol. Chem. Rapid Commu, 1986, 6: 335.
[8] Speikamp H D, Banderman F. Makromol. Chem., 1988, 189: 437.
[9] Sitz H D, Speikamp H D. Bandermann F. Makromol. Chem., 1988, 189: 429.
[10] Brittain W J, Dicker I B. Macromol., 1989, 22: 1054.