http://www.chemistrymag.org/cji/2001/034017ne.htm |
Apr. 1,
2001 Vol.3 No.4 P.17 Copyright |
Microwave high-pressure synthesis of titania nanoparticles and its resonance scattering spectrocscopic study
Jiang Zhiliang, Feng Zhongwei, Jiang Yimin,
Ling Shaoming
(Institute of New Technology and Materials, Guangxi Normal University, Guilin 541004,
China)
Received Oct. 16, 2000; Supported by the Natural Science Foundation of Guangxi and Guangxi Education Department.
Abstract Nanometer-sized TiO2
particle has been prepared by a microwave high-pressure method. The influence of
tetrabutyl titanate(TBTi) and water concentrations, microwave power and irradiation time
was investigated by resonance scattering spectroscopy and spectrophotometry. The resonance
scattering spectra shows that TiO2 nanopartcles in liquid exhibits two peaks at
350nm and 700nm. The nanoparticle size is about 14nm. This work demonstrate that microwave
high-pressure method is a good procedure for the preparation of nanoparticles in liquid,
and resonance scattering spectroscopy is a very useful technique to examine nanoparticle
property.
Keywords Microwave high-pressure synthesis, tetrabutyl titanate, titania,
nanoparticle, resonance scattering spectroscopy.
1. INTRODUCTION
Titania nanoparticles shows good chemical and physical properties. It is very interesting
to chemists, physicists, environmentalists, materialists and medical scientists[1-4].
Its synthesis, property and application have become one of focus study[1-6].
Several methods including sol-gel and hydrothermal have been reported for the synthesis of
titania nanoparticle[5]. The common shortcomings is that the formation of TiO2
particles is not simultaneous, owing to the concentration diffusion or heat conducting.
Microwave is a heating technique by means of molecular vibration in very high speed. For
fast and homogeneous advantages, this method has been utilized to many research fields[7-9].
However, microwave high-pressure procedure has not been reported for the synthesis of
titania nanoparticles and the resonance scattering spectroscopic property of the
nanopaticles in liquid has not been studied to date[10,11]. In this work,
titania nanoparticles prepared in liquid has been proposed by microwave high-pressure
method using the chemicals of tetrabutyl titanate. The resonance scattering spectroscopic
properties of the nanoparticle have been also reported.
2. EXPERIMENTAL
All chemicals are analytical grade. The tetrabutyl titanate solution was prepared as
follows: 7.0g of tetrabutyl titanate was put into a 100ml dry beaker; 3g of
triethanolamine was added to prevent hydrolysis. The solution was dissolved with alcohol,
and transferred into a 50ml volume flask, diluted to the mark with the solvent. The
microwave reactions were carried out in a microwave high-pressure reactor(the max pressure
12 atm) setting in Glanz microwave oven(Sunde China, 2450MHZ). The resonance scattering
spectra (RS), absorption spectra and TEM were obtained on a shimadiu RF-540
spectrofluorphotometer (Kyoto Japan), Hitachi U-3400 spectrophotometer (Japan) and
JEM-1200 TEM (Japan) respectively.
3. RESULTS AND DISCUSSION
The effect of water volume on the hydrolysis shows that the microwave reaction is slow
when the volume less than 0.5ml, the hydrolysis is speed up at room-temperature when the
volume is more than 5ml. However the hydrolysis is slow down at room-temperature when the
tetrabutyl titanate is less than about 10-5 mol/L.The microwave power and
irradiation time were also examined. The recommendation conditions are 3ml water-480W for
3min.
Resonance scattering ( some time also called Rayleigh ligh-scattering)
is a new spectroscopic technique. It has been applied to biochemical research, inorganic
analysis and supramolecular chemistry [10,11]. Recently this technique has been
used to estimate the properties of silver, gold and AgCl nanoparticles, (Ag)m.nAu
and (AgCl)m.nAg supramolecules in our research group[12-15] with
satisfactory results. The shape, size, absorption and rigidity of superamolecular or
nanoparticles are prime factors to affect the resonance scattering spectra. Different
shape for supramolecule or nanoparticle has the different resonance scattering spectra.
The intensity of resonance scattering is proportional to the cubic diameter of
supramolecule under the condition of the same shape[16]. Figure 1 shows the
resonance scattering spectra of titania nanoparticles.
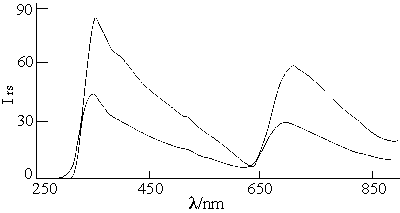
Fig. 1 The resonance scattering spectra of
titania nanoparticle
A:2.05×10-3mol/L TBTi; B:1.02×10-3mol/L TBTi
Measurement conditions:low sensitivity, ordinate scale"1"
There are two resonance scattering peaks at 350nm and 700nm. The former peak is more sensitive than the latter's. The I350nm is linear to the tetrabutyl titanate concentration in the range of 2.05×10-5-2.05×10-3mol/L. It can be explained from Rayleigh scattering formula, that is, the numbers of the nanoparticle increase with the addition of tetrabrtyl titanate. The two peaks have same properties that can be seen from the emission spectra in Figure 2. The peak at 350nm is the resonance scattering peak, the peak at 700nm is the secondary scattering peak[17, 18].
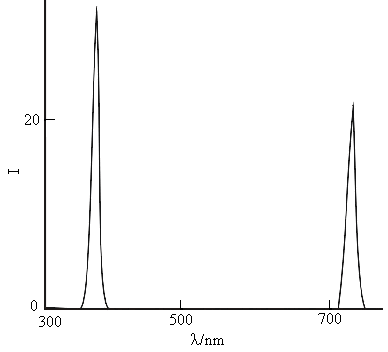
Fig.2 The emission spectra
for titania nanoparticle
lex=350nm, 1.02×10-3mol/L
TBTi
Figure 3 indicates the reagent blank(unirradiated) exhibits stronger absorption at ultraviolet region. The titania nanoparticles display absorption in the range of 370-500nm. The A400nm is proportional to the TBTi concentration in the range of 4.1×10-4-8.2×10-3mol/L. The regression coefficient is 0.998.
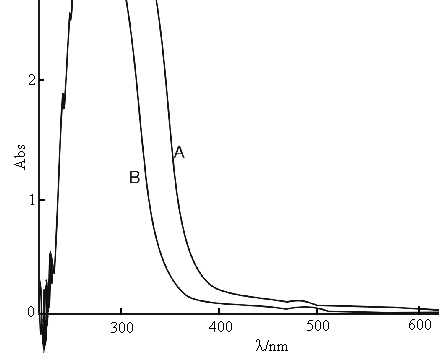 |
Fig.3
the absorption spectra for titania nanoparticle A: 4.1×10-3mol/L TBTi; B: A unirradiated by microwave |
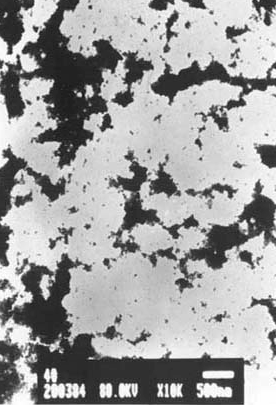 |
Fig.4 The transmission electron microscopy for titania nanoparticle |
The result of TEM (Fig.4) demonstrates that there are aggregations for titania nanoparticle owing to the nanoparticle possessing high surface energy . The mean diameter for un-aggregated TiO2 nonparticles is about 14nm. The aggregates are spongy particles,and silver ions can be embeded into the aggregates. This work is still carried on.
REFERENCES
[1] Kawa T, Sakata T. Nature, 1980, 268: 474-477.
[2] Fox M A, Dulay M Y. Chem. Rev., 1993, 93 (1): 341.
[3] Shen W R., Zhao W K, He F et al. Progress in Chemistry (Huaxue Jinzhang), 1998, 10
(4): 349-359.
[4] Luo J, Ding X Z, Cheng L F et al. Materials Science in Progress (Cailiao Kexue
Jinzhang), 1993, 7 (1): 52-57.
[5] Hug S J, Sulzberger B. Langmuir, 1994, 10: 3578-3583.
[6] Ekstrom G N, McQuillan A J. J. Phys. Chem. B, 1999, 103: 10562-10569.
[7] Bagharst R, Chippindale A M, Mingos P. Nature, 1988, 332: 311-34.
[8] Zhang H L, Liu H X, Ouyang S H. Chailiao Daobao, 1996, (4): 44-47.
[9] Mong M W, Jiang Z L. Linchang Huagong Tongsun, 1999. 21 (1): 19-23.
[10] Pasternak R F, Colling P J. Science, 1995, 269: 935-938.
[11]. Ma C Q, Liu Y, Li K A et al. Chin. Sci. Bull (Kexue Tongbao), 1999, 57: 161-166
[12] Jiang Z L, Li F, Liang H. Acta Chimica Sinica (Huaxue Xuebao), 2000, 58: 1059-1064.
[13] Pasternack R T, Colling P J, Bastamante C et al. J. Am Chem. Soc., 1993, 115:
5395-5400.
[14] Jiang Z L, Li F, Liang H. Analytical testing techniques and instruments (Fenxi Ceshi
Jishu Yu Yiqi), 2000, 6 (2): 98-101.
[15] Zhong H X, Jiang Z L, Li T S et al. Spectroscopy and spectral analysis (Guangpuxue Yu
Guangpu Fenxi), 2000, 21 (5): 746-748.
[16] Jiang Z L, Li F, Liang H. Chem. J. Chinese Universities (Gaodeng Xuexiao Huaxue
Xuebao), 2000, 21: 1488-1491.
[17] Liu S P, Liu Z F. Acta Chimica Sinica (Huaxue Xuebao), 1995, 53: 1178-1182.
[18] Huang Y Y, Jiang Z L, Wu Q D et al. J. of Anal Sci. (Fenxi Kexue Xuebao), 2000, 16
(2): 127-131.