http://www.chemistrymag.org/cji/2001/036023pe.htm |
Jun. 1,
2001 Vol.3 No.6 P.23 Copyright |
The study on the determination of protein based on the interaction between protein and complex of Cu(II)-Arsenazo K
Hu Qiuluan, Zhao Bangtun,# Shen
Yiyang
(Department of Chemistry, Luoyang Teachers College, Louyang, 471022, Henan ; # Department
of Chemistry, Nankai Universty, Tianjin 300071, China)
Keywords Serum Proteins, Cu(II)-Arsenazo K complex, Spectrophotometry. 1. INTRODUCTION
The determination of protein concentration is very important in biochemical and clinical test as well as food test. The addition of spectroscopic probe has been popularly applied in determining protein concentration. There are dye probe methods, such as bromcresol green assay [1], coomsssie brilliant blue G-250 (CBBG-250) [2], nitrosulfophenol C [3] , etc. Considering the advantages and disadvantages related to the above methods, metal complex probe of protein, which have high sensitivity and good stability, such as chromazurol S-Al(III) [4] and chromazurol S-Fe(III) [5], have been considerably noticed. The water-soluble Arsenazo K[6] (Scheme 1) are sensitive reagents for spectrophotometric determination of some metals, however, there is no report on determination of protein based on the Cu(II)-Arsenazo K-protein reaction. In the present study, we wish to report firstly that Cu(II)-Arsenazo K may reacts with proteins in acid solution to form stable ternary complex, which gives maximum absorption speak at 654 nm with 42 nm of red shift compared to that of Cu(II)- . Based on the studies of their absorption spectra characteristics, the calibration graph for determination of total protein concentration of bovine serum albumin (BSA) has been formulated. Experiments demonstrated that a new spectrophotometric analysis method for the determination of serum protein using Cu(II)-Arsenazo K complex is sensitive and accurate.
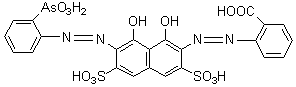
Scheme 1. The structure of aresnazok K
2. EXPERIMENTAL
2.1 Apparatus
GBC-916 UV-VIS spectrophotometer (GBC company, Australia) was used for recording the
absorption spectra, and a Model 724 spectrophotometer (Shanghai Analytical Instruments
Company, China) for the measurement of absorbance at a given wavelength, Model 821 digital
pH meter (Zhongshan University, China) was used in determining pH values.
2.2 Reagents
Arsenazo K ("R" in brief) was obtained from Beijing chemical and industrial company (China) [7]; bovine serum albumin (BSA), g-globulin (g-G), human serum albumin (HSA), hemoglobin (Hb), lysozyme (Lyso) were purchased from Sigma. All of the following reagents were of analytical grade: CuSO4 5H2O, NaCl, ethanol, glucose, citric acid, Britton-Robinson buffer solution (B-R: H3PO4-HAc-H3BO3) [8] was used to control pH values of the tested solutions. Doubly-deionized water used throughout.
2.3 Procedure
The following solutions were added to a 10 mL colorimetric tube: 1.0 mL Britton-Robinson buffer solution (pH = 2.23), 2.0 mL Arsenazo K solution, 1.0 mL standard Cu(II) solution and BSA standard solution. Then, the mixture was diluted to 10mL with doubly-deionized water to get pH = 2.82 tested solution. After laying aside for 20 min at room temperature, the spectra or the absorbance of the solution were measured with 1 cm cell at l=654 nm against corresponding agents as the blank. The determination of human serum sample solution by which was firstly diluted to 100 times was carried out as the above process.
3. RESULTS AND DISCUSSION
3.1 Absorption spectra
At pH 2.82 , the absorption spectra of Arsenazo K, Cu(II)-Arsenazo K, Arsenazo K-BSA, Cu(II)-Arsenazo K-BSA are shown in Figure 1. Their maximum absorption wavelengths (lmax) are 540nm, 612nm, 611nm and 654nm, respectively. As shown in Figure 1, red color Arsenazo K solution after the addition of Cu(II) solution was changed into violet red. The color was transformed into pure blue color after the addition of BSA to above mixture. So, this change showed that BSA may reacts with Cu(II)-Arsenazo K to form a stable ternary complex, whose lmax is 114nm longer than that of Arsenazo K and 42nm longer than that of Cu(II)-Arsenazo K. Meanwhile, without Cu(II) ion in solution, BSA may reacts with Arsenazo K to form a binary complex. Based our experimental results, the sensitivity of this binary complex (the sandell sensibility for BSA 0.35mg·cm -2) is not as good as that of this ternary complex (the sandell sensibility for BSA 0.17mg·cm -2).
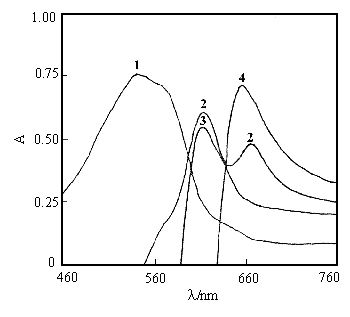
Fig. 1 Absorption spectra from 460nm to 760nm at pH 2.82 in Britton-Robinson buffer solution, where 1, 2, 3, 4 represent absorption spectra of Arsenazo K, Cu(II)-Arsenazo K, Arsenazo K-BSA, Cu(II)-Arsenazo K-BSA, respectively. (T = 298 K, CR = 1.0×10-4 mol·L-1, CCu(II) = 1.0×10-3 mol· L-1, CBSA = 1.47×10-6 mol·L-1)
3.2 Effect of pH on the reaction
As Figure 2 shown, the absorbance of Cu(
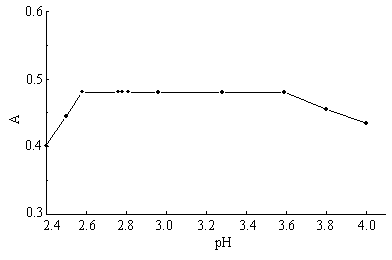 Fig. 2 pH
dependency of the absorbance of Cu(II)-Arsenazo K-BSA in
Britton-Robinson buffer solution. (l = 654 nm, T = 298 K, CR = 6.0×10-5 mol·L-1,
CCu(II) = 1.0×10-3 mol· L-1, CBSA
= 1.47×10-6 mol· L-1).
3.3 Effect of mole ratio of
Cu(II) to Arsenazo K on the absorbance
Fig. 2 pH
dependency of the absorbance of Cu(II)-Arsenazo K-BSA in
Britton-Robinson buffer solution. (l = 654 nm, T = 298 K, CR = 6.0×10-5 mol·L-1,
CCu(II) = 1.0×10-3 mol· L-1, CBSA
= 1.47×10-6 mol· L-1).
3.3 Effect of mole ratio of
Cu(II) to Arsenazo K on the absorbance In pH 2.82 solution, with the constant amount of BSA, the amount of Cu(II) or Arsenazo K was changed respectively to measure the absorbance at l = 654 nm. The absorbances at 654 nm against the ratio of Cu(II) to Arsenazo K may be seen from Figure 3 . Obviously, the absorbance reached its maximum and kept constant when the value of CCu(II)/CR was no less than 10. So, the ratio of CCu(II)/CR was kept 10 in the following experiments.
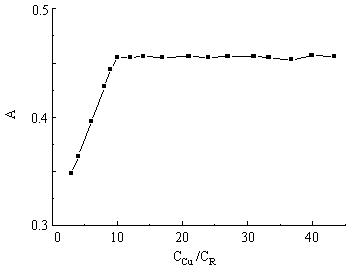
Fig 3. Effects of CCu/CR ratio on the absorbance. (pH = 2.82,
l= 654 nm, T = 298 K, CR = 6.0×10-5 mol·L-1, CBSA = 1.47×10-6 mol·L-1) 3.4 Effects of the amount of Cu(II)-Arsenazo K complex and buffer on the absorbanceIn pH 2.82 solution, with the constant amount of BSA, the amount of Cu(II)-Arsenazo K was changed to measure the absorbance. Results are shown in Figure 5. The absorbance of the systems reached its maximum and kept constant when the amount of Cu(II)-Arsenazo K was 64 times larger than that of BSA and the amount of buffer was ranged from 1.0 to 2.5mL. So, 68 times of the amount of Arsenazo K and 1.0 mL B-R buffer solution were kept in this experiment.
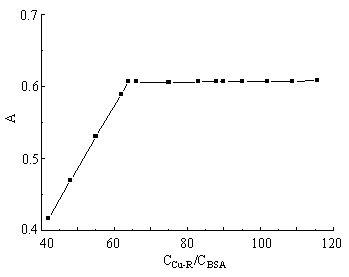
Fig. 4 Effects of CCu-R/CBSA ratio on the absorbance. (pH = 2.82, l= 654 nm, T = 298 K, CBSA = 1.47×10-6 mol·L-1)
3.5 Reaction time and temperature
dependencies
The changes of absorbance with time were tested at 298 K and 308 K (water bath),
respectively. Results demonstrated that: (1) the reaction was rapid and stable both at 298
K and at 308 K; (2) the absorbance was kept constant in 1 hour at 298 K and 308 K.
3.6 Effects of ionic strength and ethanol
The absorbance of the ternary complex system was reduced by 1.8% while NaCl was added to the final concentration 0.04mol/L, so NaCl has ignorable effect for the system. However, the absorbance of the system was increased by 4.8% when the concentration of ethanol was added to 10%(v/v), but the absorbance would be reduced by 20% if the amount of ethanol was increased by 40%.
3.7 Precision of the assay
Ten standard BSA solutions were measured, where each concentration was kept 1.47×10-6 mol·L-1, the average absorbance of system was 0.576 at l=654 nm, standard error was 1.95×10-3, RSD was 0.34%.
3.8 Calibration curves
Under the optimal conditions, the amount of proteins (BSA, HAS, Hb, g-G, Lyso) were determined, respectively, their calibration graphs are shown in Table 1. Obviously, this assay has the following outstanding characteristics: wide linear range, high sensitivity and good response to many proteins.
Table 1. Parameters for proteins determination (n = 9). ( pH = 2.82,
l = 654 nm, T = 298 K)| Proteins | Linear equations |
Linear
range |
Correlation coefficient |
e (L/mol·cm) |
S |
BSA |
A=5.68×10-3C+0.019 |
10-140 |
0.9961 |
3.86×105 |
0.17 |
HSA |
A=6.03×10-3C+0.020 |
10-140 |
0.9997 |
4.10×105 |
0.16 |
Hb |
A=4.78×10-3C+0.006 |
10-140 |
0.9991 |
3.25×105 |
0.21 |
g -G |
A=3.56×10-3C+0.002 |
10-160 |
0.9995 |
6.41×105 |
0.18 |
Lyso |
A=8.16×10-3C+0.039 |
10-140 |
0.9983 |
1.18×105 |
0.12 |
3.9 Effects of foreign substances
The influence of foreign substances, such as amino acids, metal ions, were tested. Results obtained are presented in Table 2. There are twenty basic amino acids in human body, some typical amino acids, which exist more in human serum, were tested. Experiments indicated that all of them almost do not interfere with the assay, except for glutamine, Fe3+ and Ca2+. In practical performance, the serum sample was diluted at 500 fold, therefore, interference could be ignored. Table 2. Effects of foreign substances on the determination of BSA. (pH = 2.82, l= 654 nm, T= 298 K).
| Foreign substance | Leu |
Ser |
Glu |
Lys |
Cys |
Tyr |
Pro |
Pb2+ |
Ca2+ |
Fe3+ | Cu2+ | Glucose | Citric acid |
Addition
amounts |
40 |
40 |
40 |
40 |
40 |
40 |
40 |
2 |
2 |
2 |
2 |
100 |
50 |
Error (%) |
-2.24 |
1.72 |
-4.30 |
-0.86 |
-3.10 |
-2.76 |
-0.86 |
0.00 |
-5.10 |
-5.14 |
0.40 |
0.00 |
1.32 |
Table 3. Assay results of total proteins of human serum sample
| Method | Proteins (mg/ml) |
Recovery (%) |
RSD (%) (n = 4) |
CBB G-250 |
76.7 |
2.49 |
|
Cu(II)-Arsenazo K |
76.0 |
96 |
0.68 |
Based on this method, which has a feature of similar response to many proteins, the concentration of total proteins of human serum sample was determined. Results obtained are presented in Table 3. Compared with CBB G-250 assay, this method has a good recovery and reproducibility and avoid some disadvantages of CBB G-250 method, such as, the difficulty in preparing of reagents, instability of reagents which are easy to adhere to the equipment, and the bad reproducibility of determined results.
Acknowledgment The assistance of Prof. Li Kean from the College of Chemistry and Molecular Engineering, Peking University, is gratefully acknowledged.
REFERENCES
[1] Doumas B T, Watson W A, Biggs H G. Clin. Chem. Acta, 1971, 31: 87.
[2] Bradford M M. Anal. Biochem., 1976, 72: 248.
[3] Hu Qiuluan, Li Na, Zhao Fenglin, Li Kean. Chem. J. Chinese Universities (Gaodeng
Xuexiao Huaxue Xuebao), 1999, 20 (2): 221.
[4] Saito Y, Kanetsuna A, Okuda H et al. Chem. Pharm. Bull., 1986, 34: 746.
[5] Wei Yongju, Li Kean, Tong Shenyang. Acta Chimica Sinica (Huaxue Xuebao), 1995, 53: 83.
[6] Zeng Yune, Zhang Huashan, Chen Zhenhua. The content handbok of chemical reagents
(Chinese), Beijing: Beijing Industrial University Press, 1993: 1.
[7] Beijing chemical reagent company. The content handbok of chemical reagents (Chinese),
Beijing: Beijing Industrial University Press, 1993: 516.
[8] Dean J A. Lange's Handbook of Chemistry, 12th ed., McGwraw-Hill Book Company, 1978:
5-79.