http:///www.chemistrymag.org/cji/2001/038040pe.htm |
Aug. 1,
2001 Vol.3 No.8 P.40 Copyright |
Sun Sufang, Yang Gengliang,
Wang Dexian, Sun Hanwen
(College of Chemistry and Environmental Science, Hebei University, Baoding, 071002,
China)
Received on Mar.16, 2001; Supported by the Natural Science Foundation of China (Grant No. 20075005) and the Natural Science Foundation of Hebei Province (Grant No. 200077).
Abstract The frontal velocity analysis is
a new method to be used to determine the competitive adsorption isotherms for
2-phenylethanol and 3-phenylpropanol on ODS-silica with 50:50 methanol-water as the mobile
phase. The data obtained are fitted to competitive Langmuir isotherm and the best
coefficients are acquired. Under the same conditions, a classical method, the rectangular
pulse method is also used to determine the isotherms for the same binary mixture. The
results show that the competitive isotherms obtained by the frontal velocity analysis
method are in well agreement with those obtained by the rectangular pulse method. This
shows that the frontal velocity analysis method, which is much simpler, easier and faster
than the classical methods, can be used for the determination of binary competitive
isotherms accurately.
Keywords: Competitive isotherms, Frontal velocity analysis method, Rectangular
pulse method, Liquid chromatography
The determination of an isotherm equation
that describes the distribution between mobile and stationary phase has fundamental
importance in the study of an adsorption-based separation process [1]. Although
it is commonly realized that the use of the models and the computing facilities now
available can allow considerable savings in the optimization of new separations, potential
users are still reluctant because of the experimental difficulties encountered in the
accurate determination of competitive equilibrium isotherms. Therefore, a considerable
attention is devoted to the development of new methods, which should be much more
practical and easier than the existed ones while achieving the same level of accuracy.
Several traditional methods have been used to measure competitive
isotherms, such as the static method [2], the pulse method [3-7] and
the wave method [1,8,9]. Because those methods themselves have many drawbacks,
they are not often used.
The most widely used method is the frontal analysis method [1,10-14]
rather than the above ones. There are two main variants of this method, the staircase
frontal analysis [1,10-12] and the rectangular pulse [1,13,14]. Both
methods are well suited to measure competitive isotherms of any type, but only work if the
intermediate plateau is well formed for the determination of the two concentrations on the
intermediate plateau [12,13].
In our previous papers [14,15], a new simple method of
competitive isotherm determination, the frontal velocity analysis method is presented and
used for the determination of competitive isotherms of oxazepam. The purpose of this paper
is to deal with this method for the measurement of competitive isotherms of
2-phenylethanol and 3-phenylpropanol, and compare the results with those obtained by the
classical rectangular pulse method, in order to validate the new one's
applicability in more general cases.
1 THEORETICAL
1.1 Rectangular pulse method
This method consists of injecting a single step, washing the solution off from the column
with pure mobile phase after the elution of the first step is completed, and starting
again with a new, higher step. For the binary mixture of A and B, two plateaus are
observed in the elution profiles. The first one is pure A (the less retained), and the
second one is composed of A and B, and their concentrations are the same as that of the
sample injected. The schematic is shown in Fig.1 (j=1).
According to our precious studies [14,15], the following
equations can be inferred straightforward.
Component A (the less retained):
![]() =
= 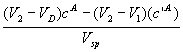 (1)
(1)
Component B (the more retained):
![]() =
=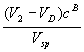 (2)
(2)
Where ![]() and
and ![]() are the concentrations of M (A or B) in the stationary phase and in the
mobile phase at equilibrium respectively.
are the concentrations of M (A or B) in the stationary phase and in the
mobile phase at equilibrium respectively. ![]() and
and
![]() are the elution volumes of the two
elution plateaus;
are the elution volumes of the two
elution plateaus; ![]() is the hold-up volume
of the column and
is the hold-up volume
of the column and ![]() is the volume of
stationary phase in the column.
is the volume of
stationary phase in the column.
Since only the first component is present during the elution of the
intermediate plateau, ![]() can be directly
derived from the height of this plateau and no analysis of the effluent is required.
can be directly
derived from the height of this plateau and no analysis of the effluent is required.
1.2 Frontal velocity analysis method
A new method called frontal velocity analysis for determining
competitive isotherm is described in the previous paper [14]. For each
concentration step of the binary solution entering the column, two concentration steps are
obtained in the elution profiles. A concentration plateau follows each step. The schematic
is shown in Fig.1.
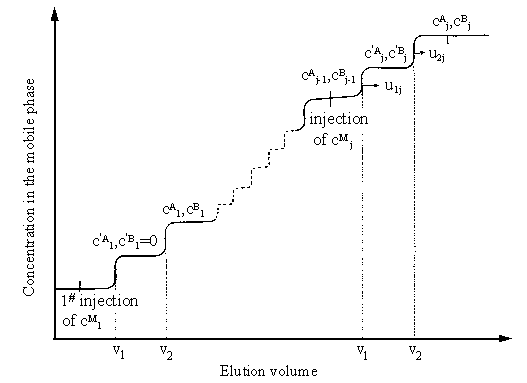
When the injection concentration step is not very high, according to reference [14], the following expressions can be inferred directly,
Where j is the rank of the concentration step;
As shown in Eqs.(3) and (4), for the frontal velocity analysis method, the competitive isotherms can be obtained by determining the retention volumes of the two fronts for every concentration step,
The only practical limitation of the method is that the concentration steps achieved should not be too high [14], and this can easily be realized in the experiment. The advantage of the frontal velocity analysis method over the classical, staircase frontal analysis method is that a second HPLC system is no longer necessary [15]. Thus, the new method is simpler and faster than conventional frontal analysis although using the same principle. Its advantage over the rectangular pulse method is to demand smaller amount of chemicals and to need less time to perform the experiments.
2 EXPERIMENTAL
2.1 Instrumentation
The schematic of the HPLC system used by the frontal velocity analysis method sees
reference[15] except that it is unnecessary to use another on-line HPLC. The
sample introduction in the frontal velocity analysis is achieved by simultaneously
switching the two valves 1 and 2, interconnected in such a way that when loop A is flushed
into the column, loop B can be filled with the solution at the next higher concentration.
The pump is Waters 510, loop A and loop B are of 2 mL volume and the injection valves are
Rheodyne 7125. The column (2.0mmi.d.×200mm) is packed with 5µm spherical ODS-silica
particles from Hypersil and it is thermostated at 35oC. The variable wavelength
UV detector is Shimadzu SPD-6AV and it is set at 275 nm in order to determine the steps
even at high concentrations. The flow rate is 0.3 mL.min-1. The signals of the
detector are recorded on a PC computer and evaluated with JS-3030 workstation.
2.2 Chemicals
2-Phenylethanol, 3-phenylpropanol and methanol are all of analytical grades from Beijing
Chemical Reagent Plant (Beijing, China). All the reagents are applied without further
purification. Doubly distilled water is used. The mobile phase is methanol-water (50:50).
Concentrations are reported in mg.mL-1: a concentration of 1mg.mL-1
is 8.2mM for 2-phenylethanol and 7.45mM for 3-phenylpropanol.
2.3 Chromatographic measurements
The column hold-up volume ![]() is measured
with methanol as the unretained marker. The volume of the stationary phase
is measured
with methanol as the unretained marker. The volume of the stationary phase ![]() is calculated from the column volume and the
dead volume. The stability of the volume flow rate of the effluent is checked periodically
by measuring the volume of column effluent collected over a measured time. The retention
volumes of the steps are calculated from the inflection points of the breakthrough curves
recorded in the two different methods. In frontal velocity analysis, first, loop A is
filled with the lowest concentration planned in the run, then injected onto the column by
simultaneously switching valves 1 and 2. Meanwhile, loop B is filled with the solution at
the next higher concentration. The corresponding new concentration step is injected at the
proper time, by turning both valves simultaneously again. Other injections required are
performed in the same way.
is calculated from the column volume and the
dead volume. The stability of the volume flow rate of the effluent is checked periodically
by measuring the volume of column effluent collected over a measured time. The retention
volumes of the steps are calculated from the inflection points of the breakthrough curves
recorded in the two different methods. In frontal velocity analysis, first, loop A is
filled with the lowest concentration planned in the run, then injected onto the column by
simultaneously switching valves 1 and 2. Meanwhile, loop B is filled with the solution at
the next higher concentration. The corresponding new concentration step is injected at the
proper time, by turning both valves simultaneously again. Other injections required are
performed in the same way.
3 RESULTS AND DISCUSSION
3.1 Experimental data
Competitive isotherm data are obtained under conditions of increasing total sample
concentration at constant mass ratios of the two components with the frontal velocity
analysis and the rectangular pulse method. The mass ratios of 2-phenylethanol (PE) to
3-phenylpropanol (PP) concentration investigated are 3:1, 1:1, and 1:3. The experimental
data are shown in Figs.2-4 (symbols) respectively. The circles represent the data obtained
from the new method and the crosses are the data from the classical method. As can be seen
in Figs.2-4 (symbols), the experimental data obtained from the frontal velocity analysis
method and the rectangular pulse method are in good agreement.
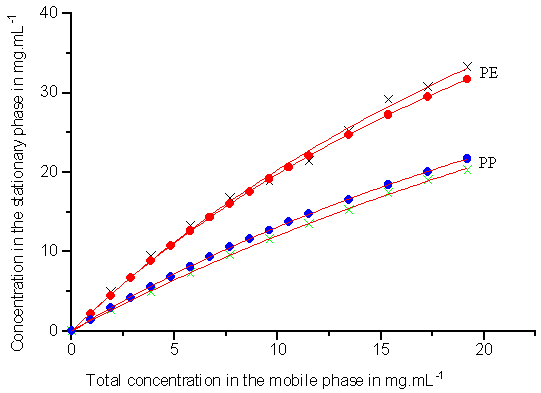
Fig.2 Competitive isotherms for 2-phenylethanol (PE) and 3-phenylpropanol (PP) (the mass ratio of PE to PP is 3:1) on ODS-silica, which are calculated by two different methods. Crosses (×) represent the data obtained from rectangular pulse method, and circles (·) are the data from frontal velocity analysis method.
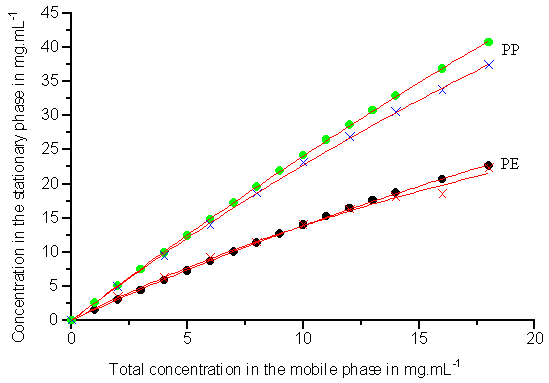
Fig.3 The same as in Fig.2, but the mass ratio of PE to PP is 1:1
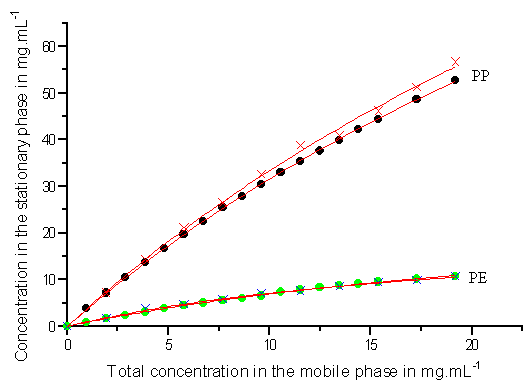
Fig.4 The same as in Fig.2, but the mass ratio of PE to PP is 1:3. 3.2 Determination of the isotherms and Coefficients of the isotherms
The experimental values obtained for the two homologues, with either method mentioned above, are fitted to the equations of the competitive Langmuir isotherms.
Where
Here
For the investigated ratios of 2-phenylethanol and 3-phenylpropanol, the experimental data obtained from the two different methods are fitted to Eqs.(7) and (8). Figs.2-4 show the competitive isotherms for PE and PP (3:1, 1:1, 1:3) on ODS-silica respectively (solid line). As can be seen in the three Figures, there is an excellent agreement between the experimental data got from the two different methods and the best Langmuir isotherms obtained from the regression. Meanwhile, the values of the four coefficients
Table 1. Langmuir constants determined by both methods.
| m(PE): m(PP) |
Frontal
Velocity Analysis Method |
Rectangular
Pulse Method |
||||||
3:1 |
2.5253 |
0.0276 |
1.5849 |
0.0210 |
2.5180 |
0.0240 |
1.3907 |
0.0157 |
1:1 |
1.5734 |
0.0134 |
2.5986 |
0.0080 |
1.7339 |
0.0250 |
2.5700 |
0.0130 |
1:3 |
0.9128 |
0.0315 |
3.8081 |
0.0205 |
1.0441 |
0.0472 |
4.0035 |
0.0199 |
* ai , bi in mL.mg-1.
3.3 Comparison of the two methods
Both the rectangular pulse and the frontal velocity analysis method are all suitable for
the measurement of any kind of isotherm if there are discontinuities in the fronts and the
same accurate results can be obtained in the experiments with different economic effects.
For the rectangular pulse method, before each individual concentration step increases, the
column must be eluted to emptiness. This is a time-consuming and solvent-wasting process.
As to the new method, the process is realized by continuous injection, i.e. the lower
concentration sample is eluted by the higher concentration sample, the solvent does not go
into the column during the whole process until the last injection is finished. Thus, the
frontal velocity analysis method is much faster and more economic than the rectangular
pulse method.
On the other hand, for the rectangular pulse method, the concentration
of the intermediate plateau can be calculated from the absorption curve of the less
retained component, this is superior to the staircase frontal analysis method, where the
concentration of the intermediate plateau is determined by another analytical HPLC system.
However, because the width of the intermediate plateau decreases with the increase of the
concentration of the two components in the mobile phase[12], when the injection
concentration is much higher, the intermediate plateau almost disappears, it is very
difficult to calculate the concentration of the component on it. While in the frontal
velocity analysis method, as seen from Eqs.(3) and (4), the only parameter needed to
determine the isotherm is the retention volume of the elution front, only does the front
shock associated with the intermediate plateau appear, the isotherms can be calculated by
the new method.
4. CONCLUSIONS
The experimental data characterizing the equilibrium adsorption for 2-phenylethanol and
3-phenylpropanol on ODS-silica are acquired by two different methods, the rectangular
pulse method and the frontal velocity analysis method. The results show that the
competitive isotherms obtained with both methods are in well agreement. This means that
the frontal velocity analysis method not only has the advantages of greater experimental
simplicity, fastness, and smaller amount of materials but also can be used to determine
binary competitive isotherms accurately.
REFERENCES
[1] Guiochon G, Golshan-Shirazi S, Katti A M. Fundamentals of Nonlinear and Preparative
Chromatography, Boston: Academic Press, 1994.
[2] Fritz W, Schluende E U. Chem. Eng. Sci., 1972, 29: 1279.
[3] Helfferich F, Peterson D L. Science, 1963, 142: 661.
[4] Stalkup F I, Kobayashi D L. AICHE J., 1963, 9: 121.
[5] Hyun S H, Danner R P. AICHE J., 1985, 31: 1077.
[6] Ruff W A, Glover C J, Watson A T. AICHE J., 1986, 32: 1948.
[7] Rouchon P, Schoenauer M, Valentin P et al. J. Phys. Chem. 1985, 89: 2076.
[8] Lin B, Ma Z, Golshan S S et al. J. Chromatogr. 1990, 500: 185.
[9] Ma Z, Katti A M, Lin B et al. J. Phys. Chem. 1990, 94: 6911.
[10] Jacobson J M, Frenz J H, Horvath Cs. Ind. Eng. Chem, Res., 1987, 26: 43-50.
[11] Jacobson J M, Frenz J H. J. Chromatogr., 1990, 499: 5-19.
[12] Ma Z, Guiochon G. Chromatogr., 1992, 603: 13.
[13] Jacobson S C, Felinger A, Guiochon G. Biotechol, Progr., 1992, 8: 533.
[14]Yang Gengliang. Chromatogr., 1999, 50: 621.
[15] Burger D, NeumÜller R, Yang Gengliang et al.
Chromatogr., 2000, 51: 517.